Transdermal selegiline is an important but highly specific treatment for adults and adolescents (12 years and older) with major depressive disorder. It is primarily reserved for patients who have not responded to other antidepressant medications (treatment-resistant depression) or for those who cannot tolerate oral medications.
While its mechanism is powerful, transdermal selegiline is considered a second or third-line therapy due to its significant interaction risks, cost, and monitoring requirements, making patient selection a critical factor for safety and success.
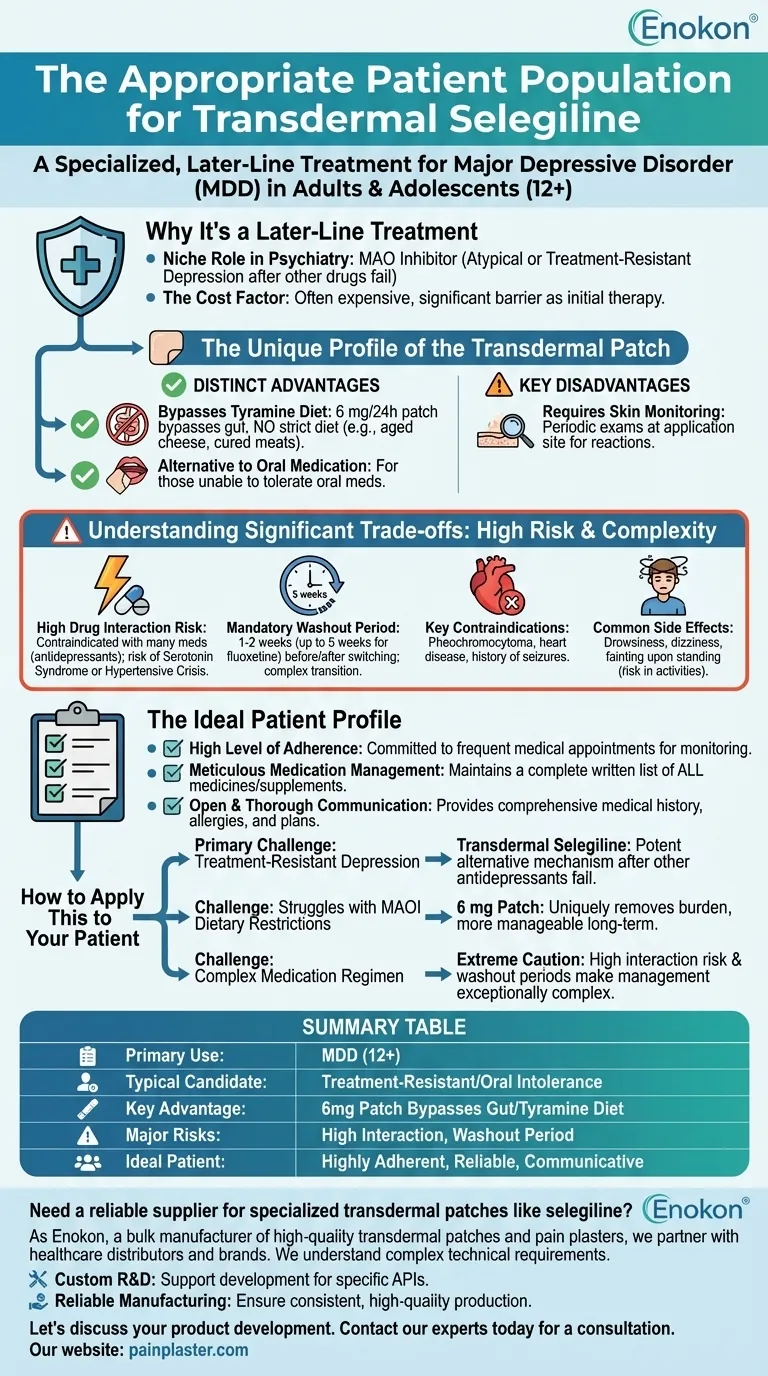
Why It's a Later-Line Treatment
Transdermal selegiline is not a first choice for most patients with depression. Its use is carefully targeted based on clinical guidelines and the patient's specific history.
A Niche Role in Psychiatry
It belongs to an older class of drugs called monoamine oxidase (MAO) inhibitors. These work by increasing the levels of brain chemicals that regulate mood.
Guidelines typically reserve MAOIs for cases of atypical depression or treatment-resistant depression after newer, safer drug classes have proven ineffective.
The Cost Factor
The transdermal patch is often an expensive option, which can be a significant barrier to its use as an initial therapy.
The Unique Profile of the Transdermal Patch
The patch delivery system offers distinct advantages and disadvantages compared to oral MAOIs, fundamentally shaping who is a good candidate for it.
Bypassing the Tyramine Diet
This is the most significant advantage. At the starting dose of 6 mg per 24 hours, the transdermal route allows the medication to bypass the gut, eliminating the need for the strict, tyramine-restricted diet required for most oral MAOIs.
This is a major quality-of-life improvement, as the traditional MAOI diet forbids foods like aged cheeses, cured meats, and some fermented products to prevent a hypertensive crisis.
An Alternative to Oral Medication
The patch is a viable solution for patients who have difficulty with or cannot take oral medications for any reason.
Requires Skin Monitoring
A unique requirement of the patch is the need for periodic skin examinations at the application site to monitor for any adverse reactions.
Understanding the Significant Trade-offs
The benefits of transdermal selegiline must be weighed against its considerable risks and management complexities. It demands a high level of caution from both the clinician and the patient.
High Risk of Drug Interactions
This is the most critical safety concern. Selegiline is contraindicated with many other medications, especially other antidepressants.
Combining it with certain drugs can lead to life-threatening conditions like serotonin syndrome or a hypertensive crisis.
The Necessity of a "Washout" Period
When switching from another antidepressant, a washout period is mandatory. This can be 1-2 weeks for most drugs but extends to five weeks after discontinuing fluoxetine. This complex transition requires careful planning and patient adherence.
Key Medical Contraindications
Patients with certain medical conditions, such as pheochromocytoma (a rare adrenal tumor), heart disease, or a history of seizures, may not be suitable candidates.
Common Side Effects
The medication may cause drowsiness, dizziness, or fainting upon standing up quickly. Patients must be counseled on these risks, especially regarding activities like driving.
The Ideal Patient Profile
A successful outcome with transdermal selegiline depends heavily on the patient's ability to partner in their own care. The ideal candidate is well-informed and highly reliable.
High Level of Adherence
The patient must be committed to frequent medical appointments, especially at the beginning of treatment, for close monitoring.
Meticulous Medication Management
The patient must maintain a complete, written list of all medicines, including over-the-counter drugs and herbal supplements. This list must be shared with every healthcare provider to prevent dangerous interactions.
Open and Thorough Communication
The patient must be able to provide a comprehensive medical history, including all allergies, past and present conditions, and plans for pregnancy or surgery.
How to Apply This to Your Patient
Choosing this therapy requires a clear understanding of the primary treatment goal and the patient's capacity to manage the associated risks.
- If the primary challenge is treatment-resistant depression: Transdermal selegiline offers a potent alternative mechanism of action when multiple other antidepressants have failed.
- If the patient struggles with MAOI dietary restrictions: The 6 mg patch uniquely removes this significant burden, making it a more manageable long-term option than oral MAOIs.
- If the patient has a complex medication regimen: Extreme caution is required, as the high risk of severe drug interactions and the need for lengthy washout periods make management exceptionally complex.
Ultimately, transdermal selegiline is a specialized tool that, for the right, carefully selected patient, can provide profound relief where other treatments have not.
Summary Table:
| Key Consideration | Details |
|---|---|
| Primary Use | Major Depressive Disorder (MDD) in adults & adolescents (12+) |
| Typical Candidate | Treatment-resistant depression or intolerance to oral medications |
| Key Advantage | 6mg/24h patch bypasses gut, often eliminating strict tyramine diet |
| Major Risks | High risk of drug interactions (e.g., serotonin syndrome), requires washout period |
| Ideal Patient | Highly adherent, reliable with medication management and communication |
Need a reliable supplier for specialized transdermal patches like selegiline?
As Enokon, a bulk manufacturer of high-quality transdermal patches and pain plasters, we partner with healthcare and pharmaceutical distributors and brands. We understand the precise technical requirements for complex drug delivery systems.
Benefit from our expertise:
- Custom R&D: We can support the development of patches for specific APIs and patient needs.
- Reliable Manufacturing: Ensure consistent, high-quality production for your specialized formulations.
Let's discuss how we can support your transdermal product development. Contact our experts today for a consultation.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Herbal Eye Protection Patch Eye Patch
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Menthol Gel Pain Relief Patch
People Also Ask
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health















