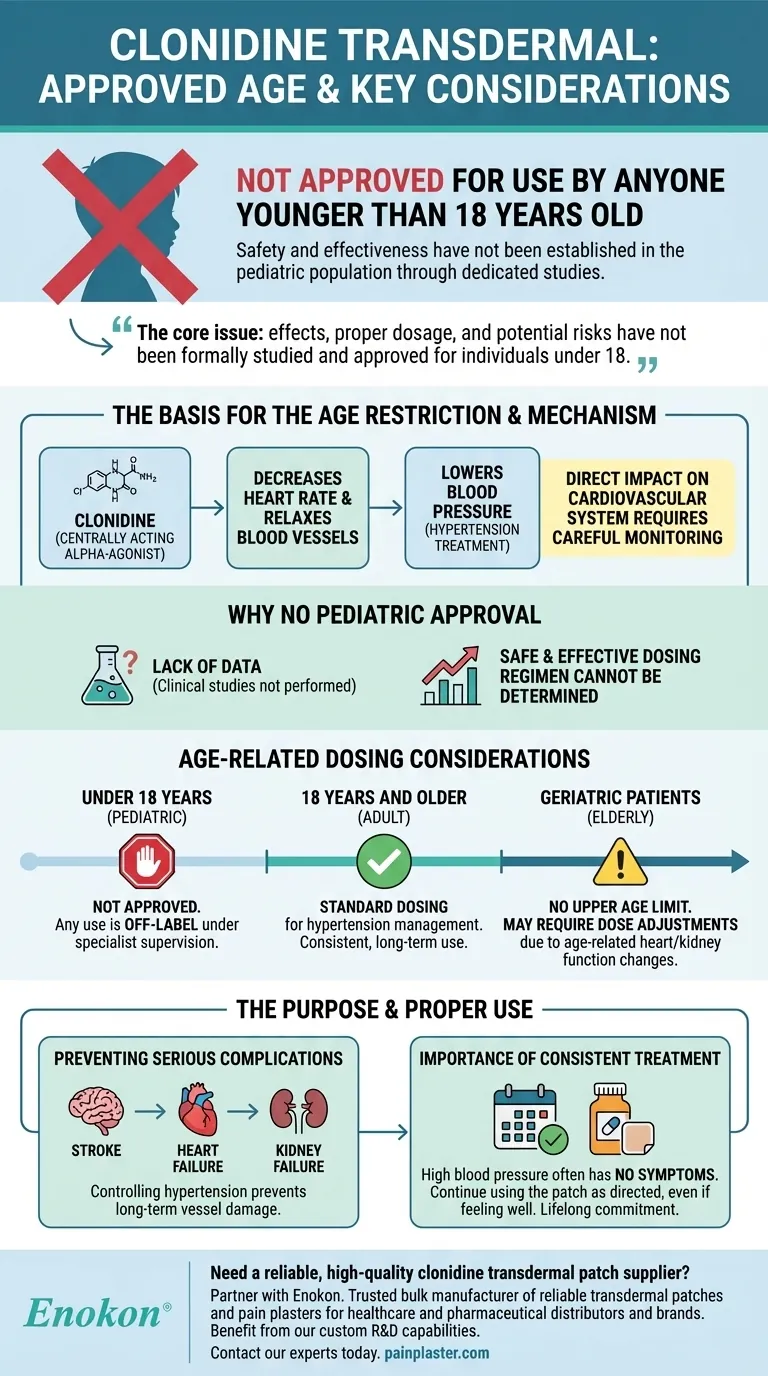
To be clear, the clonidine transdermal patch is not approved for use by anyone younger than 18 years old. The safety and effectiveness of this medication have not been established in the pediatric population through dedicated studies.
The core issue is not that clonidine transdermal is definitively unsafe for children, but rather that its effects, proper dosage, and potential risks have not been formally studied and approved for individuals under 18.
The Basis for the Age Restriction
Clonidine is a powerful medication used to treat high blood pressure (hypertension). Its mechanism requires careful management, and age is a critical factor in determining its appropriate use.
Understanding the Mechanism of Action
Clonidine belongs to a class of drugs called centrally acting alpha-agonist hypotensive agents. It works by decreasing the heart rate and relaxing blood vessels, which allows blood to flow more easily and lowers blood pressure.
This direct impact on the cardiovascular system is why its use must be carefully monitored by a healthcare professional.
Why It Is Not Approved for Pediatrics
The primary reason for the age restriction is a lack of data. Safety and efficacy have not been established for pediatric patients because appropriate clinical studies have not been performed in this age group.
Without these studies, a safe and effective dosing regimen cannot be determined, and the full scope of potential side effects in children is unknown.
Age-Related Dosing Considerations
While the focus is on the lower age limit, it's also important to understand how age can influence treatment at the other end of the spectrum.
The Official Stance on Pediatric Use
As stated, clonidine transdermal is not approved for anyone younger than 18 years old. Any use in this population would be considered "off-label" and should only occur under the direct supervision of a specialist.
Important Notes for Geriatric Patients
For elderly patients, there is no specific upper age limit. However, they may be more sensitive to the effects of clonidine.
Physicians may need to adjust the dose for older adults, particularly those with age-related heart or kidney problems that can affect how the body processes the medication.
The Purpose and Proper Use of Clonidine
Understanding the "why" behind the prescription is crucial for patient adherence and safety.
Preventing Serious Health Complications
Clonidine transdermal is used alone or with other medicines to treat high blood pressure. Controlling hypertension is critical for preventing long-term damage to blood vessels in the brain, heart, and kidneys, which can lead to stroke, heart failure, or kidney failure.
The Importance of Consistent Treatment
High blood pressure often has no symptoms. For this reason, it is essential to continue using the clonidine patch as directed, even if you feel perfectly well. For many, blood pressure medication is a lifelong commitment to prevent future health crises.
How to Apply This to Your Situation
Your approach to this information depends on your specific role and concern.
- If you are a parent or guardian: Understand that clonidine transdermal is not approved for children under 18 due to a lack of specific safety and efficacy studies in this population.
- If you are an adult patient: This medication is designed for consistent, long-term use to manage hypertension and should be used exactly as prescribed by your doctor.
- If you are an elderly patient or caregiver: Be aware that dose adjustments may be necessary to account for age-related changes in heart or kidney function, and maintain open communication with your healthcare provider.
Always consult with a qualified healthcare professional for medical advice tailored to your specific health needs.
Summary Table:
| Age Group | Approved for Use? | Key Consideration |
|---|---|---|
| Under 18 years | No | Safety and efficacy not established; use is off-label. |
| 18 years and older | Yes | Standard dosing for hypertension management. |
| Geriatric Patients | Yes | Dose adjustments may be needed due to age-related health changes. |
Need a reliable, high-quality clonidine transdermal patch supplier?
Partner with Enokon, a trusted bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharmaceutical distributors and brands. We provide the consistent quality and technical expertise necessary for complex medications. Benefit from our custom R&D capabilities to develop your next-generation transdermal solution.
Contact our experts today to discuss your manufacturing needs.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Herbal Eye Protection Patch Eye Patch
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
















