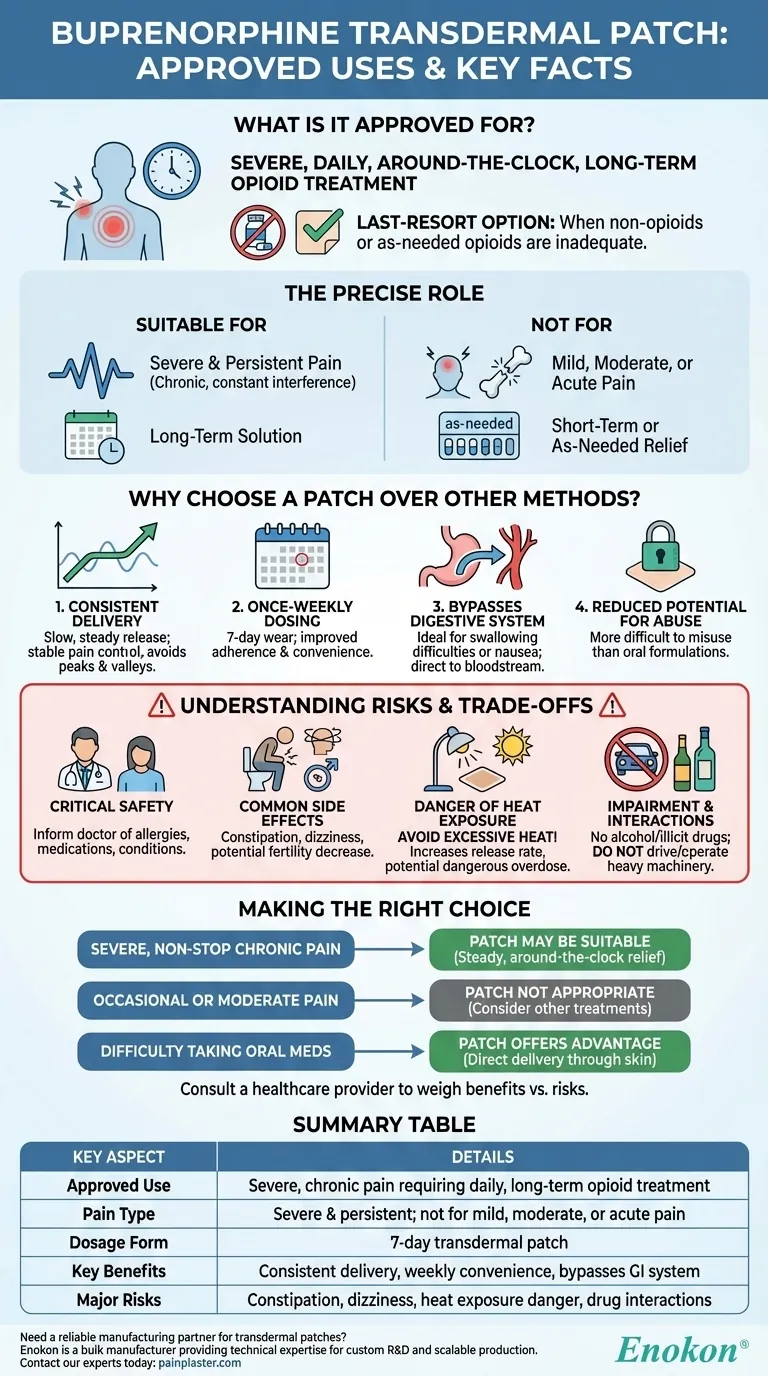
The buprenorphine transdermal patch is approved for a very specific type of pain management. It is indicated for patients experiencing pain severe enough to require daily, around-the-clock, long-term opioid treatment. This medication is reserved for situations where other treatment options, such as non-opioid pain relievers or as-needed opioids, are not adequate to manage the pain.
The core purpose of the buprenorphine patch is to provide continuous, background pain relief for severe chronic conditions. It is not designed for mild, short-term, or occasional pain, but rather as a long-term solution when other methods have failed.

The Precise Role of the Buprenorphine Patch
To understand the patch's role, it's crucial to break down its specific indication. It is a highly specialized tool, not a general-purpose pain reliever.
Defining "Severe and Persistent" Pain
This medication is intended for chronic pain that is constant and significantly interferes with daily life. It is not for treating mild or moderate pain, such as a headache or a minor injury.
A Last-Resort Opioid Treatment
The buprenorphine patch is prescribed only when other pain management strategies have been exhausted. This includes non-opioid medications and short-acting opioids, which may have been ineffective or caused intolerable side effects.
Not for Short-Term or As-Needed Relief
Unlike pills taken as pain arises, the patch delivers a steady dose of medication over seven days. It is entirely unsuitable for acute pain or pain that can be controlled with medication taken only when needed.
Why Choose a Patch Over Other Methods?
The decision to use a transdermal patch instead of oral medications is based on several distinct advantages that address specific patient needs.
Consistent Medication Delivery
The patch releases buprenorphine at a slow, steady rate through the skin. This provides consistent blood levels of the medication, avoiding the peaks and valleys often associated with taking pills, which can lead to better, more stable pain control.
Convenience of Once-Weekly Dosing
A single patch is worn for seven days, offering significant convenience over medications that must be taken multiple times a day. This can greatly improve a patient's ability to adhere to their treatment plan.
Bypassing the Digestive System
For patients who have difficulty swallowing pills or who experience significant nausea and vomiting from oral medications, the transdermal patch is an ideal alternative. The drug is absorbed directly into the bloodstream, bypassing the gastrointestinal tract.
Reduced Potential for Abuse
While not impossible to misuse, substances delivered via a transdermal patch are generally considered more difficult to abuse than traditional oral formulations. This can be an important safety factor in long-term opioid therapy.
Understanding the Risks and Trade-offs
Like any opioid, the buprenorphine patch carries significant risks and requires careful management under a doctor's supervision.
Critical Safety Considerations
Before starting treatment, you must inform your doctor of any allergies, all other medications you are taking, and any pre-existing medical conditions. This includes heart disease, seizure disorders, or liver problems.
Common Side Effects
Patients may experience side effects such as constipation, dizziness (especially when standing up quickly), and potential decreases in fertility. It is crucial not to drive or operate heavy machinery until you know how the medication affects you.
The Danger of Heat Exposure
You must avoid exposing the patch to excessive heat from sources like heating pads, electric blankets, hot tubs, or prolonged direct sun. Heat can increase the rate at which the medication is released, potentially causing a dangerous overdose.
Impairment and Interactions
The use of alcohol or illicit drugs while using the buprenorphine patch can lead to severe side effects, including respiratory distress and death.
Making the Right Choice for Your Goal
The decision to use the buprenorphine patch must be made in close consultation with a healthcare provider who can weigh the benefits against the risks for your specific condition.
- If your primary focus is managing severe, non-stop chronic pain: The patch may be a suitable option for providing the steady, around-the-clock relief that other medications cannot.
- If your primary focus is treating occasional or moderate pain: This medication is not appropriate; other as-needed or non-opioid treatments should be your first consideration.
- If you have difficulty taking oral medications due to swallowing issues or nausea: The transdermal patch offers a significant advantage by delivering medication directly through the skin.
Ultimately, the buprenorphine patch is a specialized tool reserved for a challenging and specific pain scenario where its continuous delivery provides a clear benefit over other available options.
Summary Table:
| Key Aspect | Details |
|---|---|
| Approved Use | Severe, chronic pain requiring daily, around-the-clock, long-term opioid treatment |
| Pain Type | Severe & persistent; not for mild, moderate, or acute pain |
| Dosage Form | 7-day transdermal patch |
| Key Benefits | Consistent delivery, weekly convenience, bypasses GI system |
| Major Risks | Constipation, dizziness, heat exposure danger, drug interactions |
Need a reliable manufacturing partner for buprenorphine or other transdermal patches?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we provide healthcare and pharma distributors and brands with the technical expertise for custom R&D and development. Benefit from our consistent quality and scalable production to bring your specialized pain management solutions to market.
Contact our experts today to discuss your project requirements.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Icy Hot Menthol Medicine Pain Relief Patch
- Menthol Gel Pain Relief Patch
People Also Ask
- How should pain relief patches be applied and used? A Guide to Safe & Effective Targeted Relief
- How do pain relief patches compare to other pain relief methods? Discover Targeted, Long-Lasting Relief
- What are pain relief patches and how are they used? A Guide to Safe, Targeted Relief
- How effective are pain relief patches for muscle pain? Target Localized Pain with Transdermal Delivery
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
















