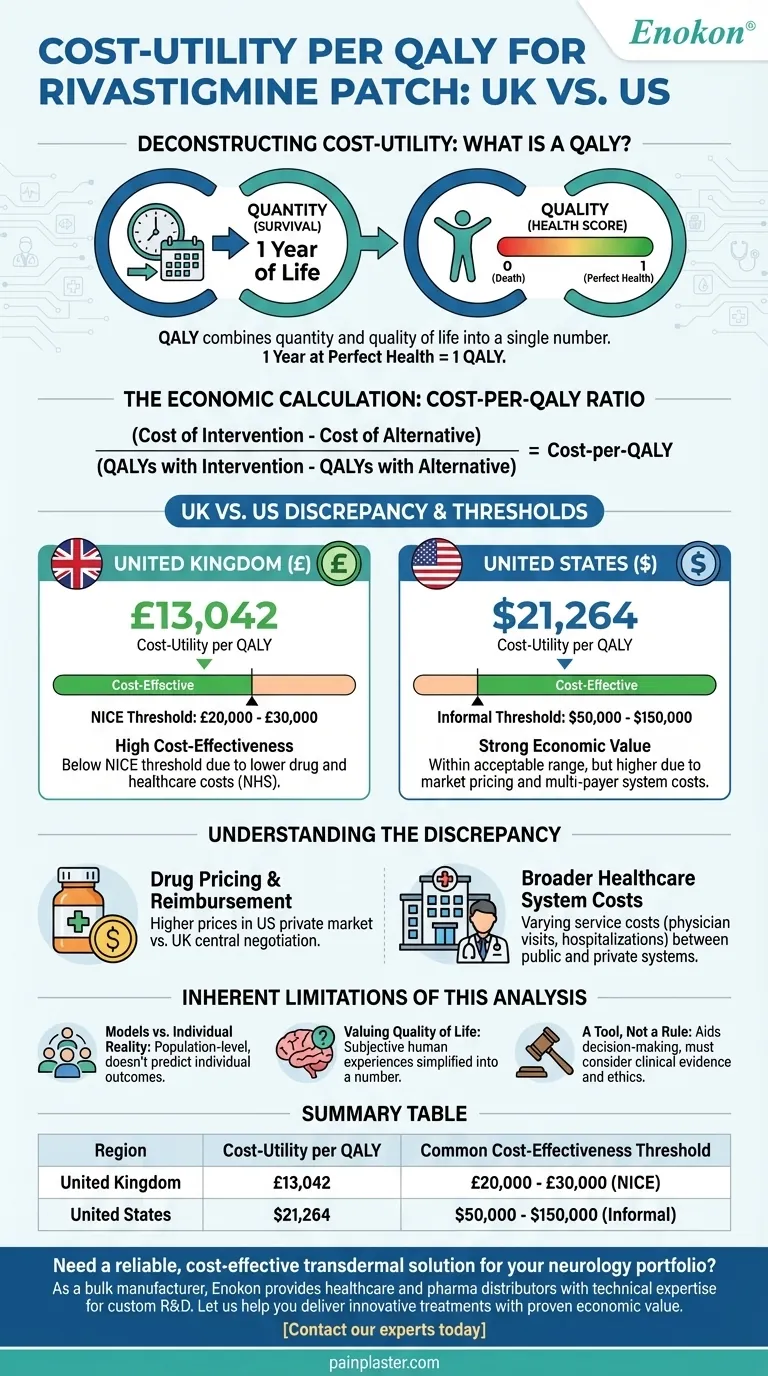
Based on the available data, the cost-utility per quality-adjusted life year (QALY) for the rivastigmine patch is £13,042 in the United Kingdom and $21,264 in the United States. These figures represent the incremental cost of achieving one extra year of perfect health by using the patch compared to alternative treatments or no treatment.
The core takeaway is that while the rivastigmine patch is considered cost-effective in both the UK and the US, its specific value is judged against entirely different economic thresholds and healthcare system structures. Understanding this context is more important than the raw numbers themselves.

Deconstructing Cost-Utility: What is a QALY?
The Goal: A Universal Metric for Health
Health economics requires a standardized unit to compare the value of different medical interventions, from a new cancer drug to a surgical procedure. The Quality-Adjusted Life Year (QALY) is the most widely accepted metric for this purpose.
It combines two critical outcomes into a single number: the quantity of life (survival) and the quality of life.
The "Quality" Component
Quality of life is measured on a scale from 1 (perfect health) to 0 (a state judged equivalent to death). A treatment that improves a patient's daily function and reduces symptoms, even without extending their life, can generate QALYs by increasing their quality-of-life score.
The "Life Year" Component
One year of life lived in a state of perfect health is equal to 1 QALY (1 year x 1.0 quality score). A year lived with a chronic condition that reduces quality of life to, for example, a score of 0.7 would be equivalent to 0.7 QALYs.
The Economic Calculation: Is It "Worth It"?
How the Cost-per-QALY Ratio is Calculated
The cost-utility figure is a ratio. It is calculated by dividing the net increase in healthcare costs of an intervention (like the rivastigmine patch) by the net health gain in QALYs it provides.
Cost-per-QALY = (Cost of Intervention - Cost of Alternative) / (QALYs with Intervention - QALYs with Alternative)
A lower number indicates greater cost-effectiveness; you are paying less for each unit of health gained.
The "Willingness-to-Pay" Threshold
The resulting cost-per-QALY figure is then compared against a country's "willingness-to-pay" threshold. This is a benchmark, often implicit or explicit, that a healthcare system uses to decide if an intervention offers good value for money.
In the UK, the National Institute for Health and Care Excellence (NICE) generally uses a threshold of £20,000 to £30,000 per QALY. Because the rivastigmine patch's figure of £13,042 falls comfortably below this range, it is typically deemed cost-effective.
The US does not have a formal, centralized threshold, but figures between $50,000 to $150,000 per QALY are often cited as acceptable in academic and policy discussions. The patch's $21,264 figure falls well within this range.
Understanding the UK vs. US Discrepancy
Factor 1: Drug Pricing and Reimbursement
The fundamental reason for the different cost-utility figures is the difference in the underlying costs within each country's healthcare system. Pharmaceutical prices are often significantly higher in the US than in the UK, where the government negotiates prices centrally for the National Health Service (NHS).
Factor 2: Broader Healthcare System Costs
The calculation also includes other costs, such as physician visits, hospitalizations, and caregiver support. The prices for these services vary dramatically between the public, single-payer system in the UK and the private, multi-payer system in the US.
These two factors mean that while the health gain (the QALYs) from the patch may be similar in both countries, the cost to achieve that gain is different, leading to distinct cost-utility ratios.
The Inherent Limitations of This Analysis
Models vs. Individual Reality
A cost-per-QALY analysis is a population-level economic model. It provides guidance for policy and resource allocation but cannot predict the value or outcome for a specific individual patient.
The Challenge of Valuing Quality of Life
Assigning a single numerical value to a person's quality of life is inherently subjective and can be controversial. These models often simplify complex human experiences and may not capture all aspects of a treatment's benefit, such as caregiver relief or patient dignity.
A Tool for Decision-Making, Not a Rule
These figures are powerful tools for making rational, evidence-based decisions about healthcare spending on a large scale. However, they are not absolute rules and should always be considered alongside clinical evidence, patient preference, and ethical considerations.
How to Interpret These Figures for Your Goal
- If your primary focus is public health policy (e.g., in the UK): The £13,042 figure indicates the rivastigmine patch is a highly cost-effective intervention and represents good value for NHS resources compared to the established NICE threshold.
- If your primary focus is formulary decisions in the US: The $21,264 figure suggests the patch offers strong economic value, likely justifying its inclusion on a list of approved medications for a health plan or hospital system.
- If your primary focus is clinical practice: This data supports the use of the patch from an efficiency standpoint, but your decision should always be driven by the individual patient's clinical needs, potential side effects, and personal circumstances.
Ultimately, cost-utility analysis provides a vital economic lens to help ensure that healthcare resources are used wisely to maximize health for the population.
Summary Table:
| Region | Cost-Utility per QALY | Common Cost-Effectiveness Threshold |
|---|---|---|
| United Kingdom | £13,042 | £20,000 - £30,000 (NICE) |
| United States | $21,264 | $50,000 - $150,000 (Informal) |
Need a reliable, cost-effective transdermal solution for your neurology portfolio?
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon provides healthcare and pharma distributors with the technical expertise for custom R&D and development. Let us help you deliver innovative treatments with proven economic value.
Contact our experts today to discuss your custom transdermal patch needs.
Visual Guide
Related Products
- Herbal Eye Protection Patch Eye Patch
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Pain Patch Relief Pain Reliever for Back
- Far Infrared Knee Pain Patch Heat Patches for Pain Relief
- Prostate Pain Kidney Health Care Patch for Men
People Also Ask
- How can using eye patches contribute to a self-care skincare routine? Boost Hydration & Relaxation
- Should under eye patches be applied before or after moisturizer? Optimize Your Skincare Routine
- How do eye patches enhance the effectiveness of eye creams? Boost Your Eye Care Routine
- What are the steps for applying under-eye patches? Boost Your Eye Care Routine
- What factors should be considered when purchasing eye patches? Essential Guide for Safe & Effective Use













