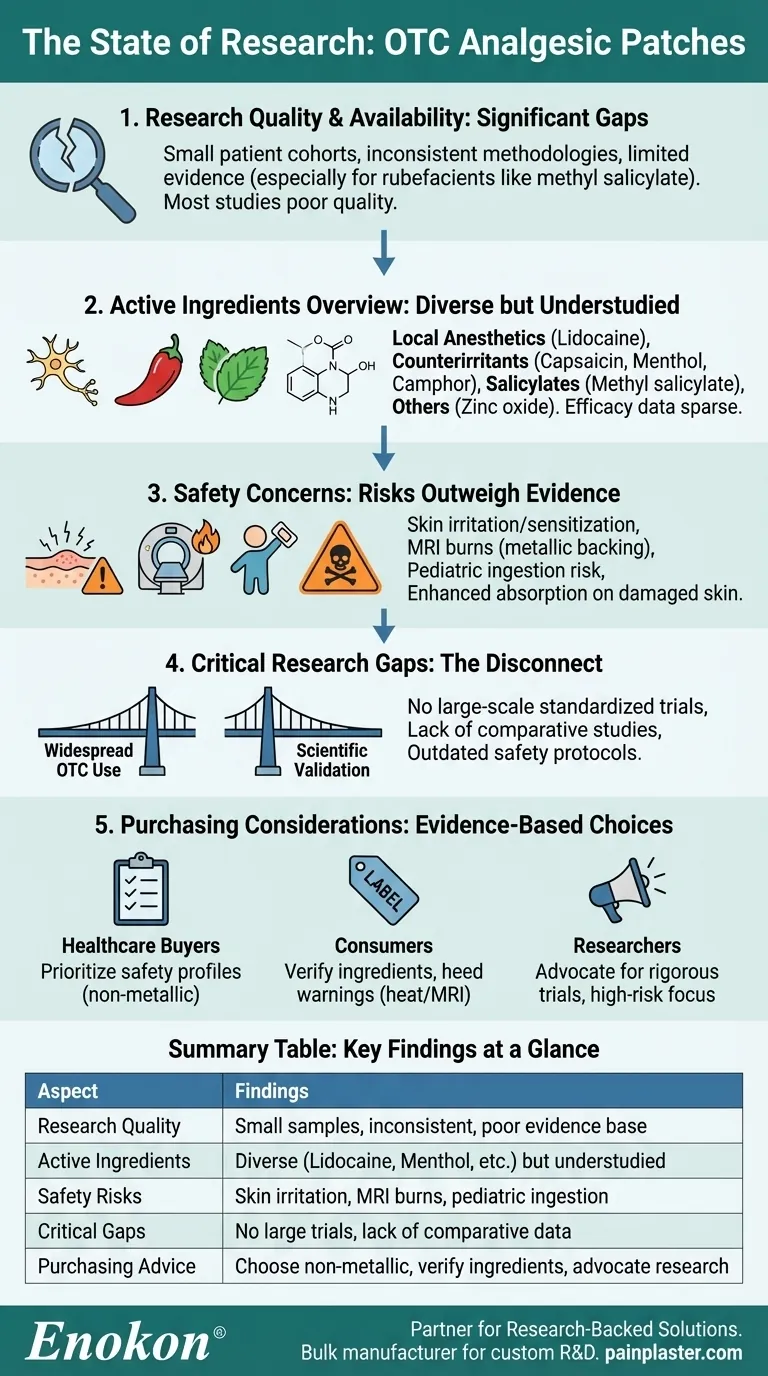
Current research on OTC analgesic patches reveals significant gaps in evidence quality and quantity. Most studies suffer from small sample sizes, inconsistent methodologies, and limited data supporting common ingredients like methyl salicylate. While these patches contain diverse active compounds (lidocaine, capsaicin, menthol, etc.), their efficacy remains understudied compared to risks such as skin irritation, burns during MRI scans, or accidental pediatric ingestion. The field urgently needs larger, standardized trials to validate pain relief claims and establish safety protocols, especially for vulnerable populations.

Key Points Explained:
-
Research Quality and Availability
- Multiple references highlight that studies on OTC analgesic patches are "lacking and of poor quality."
- Common limitations include:
- Small patient cohorts
- Inconsistent study designs
- Minimal evidence for rubefacients (e.g., methyl salicylate), a frequent active ingredient.
-
Active Ingredients Overview
- Patches contain varied compounds targeting pain relief:
- Local anesthetics: Lidocaine (up to 4%)
- Counterirritants: Capsaicin (0.002%-3.75%), menthol (0.2%-16%), camphor (0.13%-11%)
- Salicylates: Methyl salicylate (0.04%-20%), glycol salicylate (4.66%)
- Other: Zinc oxide, sulfur, tocopherol acetate.
- Despite this diversity, efficacy data for most ingredients remain sparse.
- Patches contain varied compounds targeting pain relief:
-
Safety Concerns
- Skin reactions: Irritation or sensitization is common.
-
Serious risks:
- Metallic-backed patches may cause burns during MRI or heat exposure.
- Residual drug in used patches poses ingestion risks to children.
- Enhanced absorption in patients with damaged skin barriers.
- Pediatric toxicity: Accidental ingestion can lead to acute poisoning.
-
Critical Research Gaps
- No large-scale, standardized trials validate pain relief claims.
- Limited comparative studies between ingredients or formulations.
- Safety protocols (e.g., MRI warnings, pediatric storage) need evidence-based updates.
-
Purchasing Considerations
- For healthcare buyers: Prioritize patches with clearer safety profiles (e.g., non-metallic backing).
- For consumers: Verify ingredient concentrations and heed warnings about heat/MRI exposure.
- For researchers: Advocate for rigorous trials focusing on high-risk populations and real-world usage patterns.
The current state of research underscores a disconnect between widespread OTC use and scientific validation. Future studies should address these gaps to ensure safer, more effective pain management options.
Summary Table:
| Key Aspect | Findings |
|---|---|
| Research Quality | Limited studies with small samples, inconsistent methods, poor evidence base. |
| Active Ingredients | Lidocaine, capsaicin, menthol, salicylates—diverse but understudied. |
| Safety Risks | Skin irritation, MRI burns, pediatric ingestion hazards. |
| Critical Gaps | No large trials, lack of comparative data, outdated safety protocols. |
| Purchasing Advice | Choose non-metallic patches; verify ingredients; advocate for better research. |
Need reliable, research-backed analgesic patches? Partner with Enokon, a bulk manufacturer of transdermal pain relief solutions for healthcare distributors and brands. Benefit from our technical expertise in custom R&D to develop safer, evidence-based formulations. Contact us today to discuss your needs!
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- What are pain relief patches and how are they used? A Guide to Safe, Targeted Relief
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- How effective are pain relief patches for muscle pain? Target Localized Pain with Transdermal Delivery

















