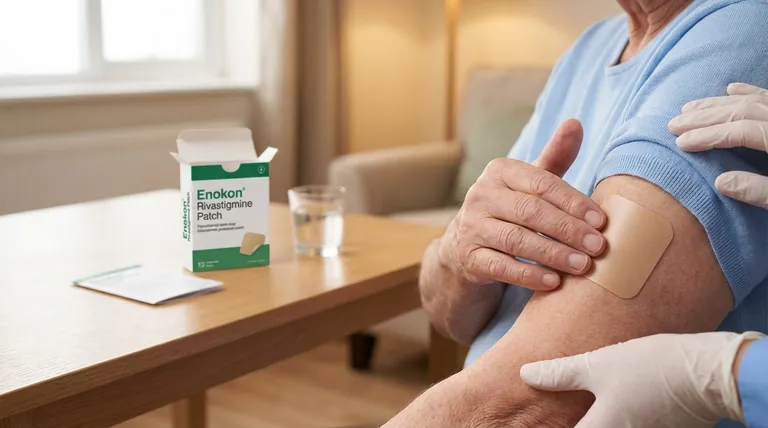
To be clear, the rivastigmine transdermal patch is approved in the United States for treating dementia associated with Parkinson's disease (PDD). This approval was granted based on data demonstrating that the patch is bioequivalent to the capsule form, which had already proven its efficacy in this patient population.
The rivastigmine patch is considered a clinically valuable and approved treatment for Parkinson's disease dementia because it delivers the same therapeutic benefits as the proven oral capsule but in a more convenient transdermal form.
The Basis for Approval: Bridging from Capsules to Patch
Understanding the approval pathway for the rivastigmine patch reveals why it's considered a trusted therapeutic option. The process didn't start from scratch; it built upon existing knowledge of the drug.
What is Bioequivalence?
Bioequivalence is a regulatory term indicating that a different formulation of a drug (like a patch versus a capsule) delivers the same amount of the active ingredient into the bloodstream over the same period.
Essentially, it proves the body sees and processes the drug from the patch in the same way it does from the capsule.
Leveraging Proven Efficacy
The oral capsule form of rivastigmine had already undergone rigorous trials and demonstrated its effectiveness in treating PDD.
By proving the patch was bioequivalent to the capsule, regulators could confidently infer that the patch would provide the same clinical benefits without requiring a full new set of large-scale efficacy trials.
The Clinical Value of the Transdermal Patch
Approval is one thing; clinical utility is another. The patch provides distinct benefits in the management of dementia symptoms.
Improvements in Core Symptoms
Clinical data confirms the rivastigmine patch improves key outcomes for patients. These include measurable benefits in cognition, overall clinical impression, and the ability to perform activities of daily living.
Practical Advantages of a Patch
The transdermal system offers a steady, continuous release of medication through the skin. This can lead to more stable drug levels in the body compared to the peaks and troughs of oral dosing.
This delivery method is often easier for patients who have difficulty swallowing pills and simplifies the medication regimen for caregivers.
Understanding the Ongoing Safety Evaluation
Regulatory approval is not the end of the story for any medication. The commitment to patient safety continues long after a product is on the market.
The Purpose of Post-Approval Studies
It is standard practice for regulatory agencies to require ongoing monitoring and studies even after a drug is approved. This helps gather more long-term, real-world data.
Focus on Safety in PDD Patients
For the rivastigmine patch, a specific safety-focused study in patients with Parkinson's disease dementia is currently ongoing.
This demonstrates a commitment to ensuring the long-term safety profile of the patch is well-understood specifically within this distinct patient group.
Making an Informed Treatment Decision
The rivastigmine patch is an established tool, not an experimental one. Choosing it depends on specific patient needs and treatment goals.
- If your primary focus is established efficacy: The patch is a valid choice, as its approval is directly linked to the proven effectiveness of oral rivastigmine.
- If your primary focus is treatment adherence and ease of use: The patch offers a clear advantage over oral capsules, particularly for patients with swallowing difficulties or for simplifying daily medication management.
Ultimately, the rivastigmine patch represents a valuable and approved therapeutic option for managing the cognitive symptoms of Parkinson's disease dementia.
Summary Table:
| Key Aspect | Status/Detail |
|---|---|
| FDA Approval Status | Approved for Parkinson's Disease Dementia (PDD) |
| Approval Basis | Bioequivalence to the proven oral capsule |
| Clinical Benefits | Improves cognition, daily living activities, and overall clinical impression |
| Key Advantage | Convenient transdermal delivery for better adherence |
| Ongoing Monitoring | Safety-focused studies in PDD patients continue |
Partner with Enokon for Your Transdermal Patch Development
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon provides healthcare and pharmaceutical distributors and brands with the technical expertise needed for custom R&D and development. If you are considering developing a transdermal solution for your therapeutic area, leverage our experience to ensure quality, efficacy, and patient convenience.
Contact our experts today to discuss your project requirements.
Visual Guide
Related Products
- Herbal Eye Protection Patch Eye Patch
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- What factors should be considered when purchasing eye patches? Essential Guide for Safe & Effective Use
- What are the main benefits of using eye patches in a skincare routine? Revitalize Your Under-Eye Area
- How do eye patches enhance the effectiveness of eye creams? Boost Your Eye Care Routine
- Should under eye patches be applied before or after moisturizer? Optimize Your Skincare Routine
- What are the steps for applying under-eye patches? Boost Your Eye Care Routine














