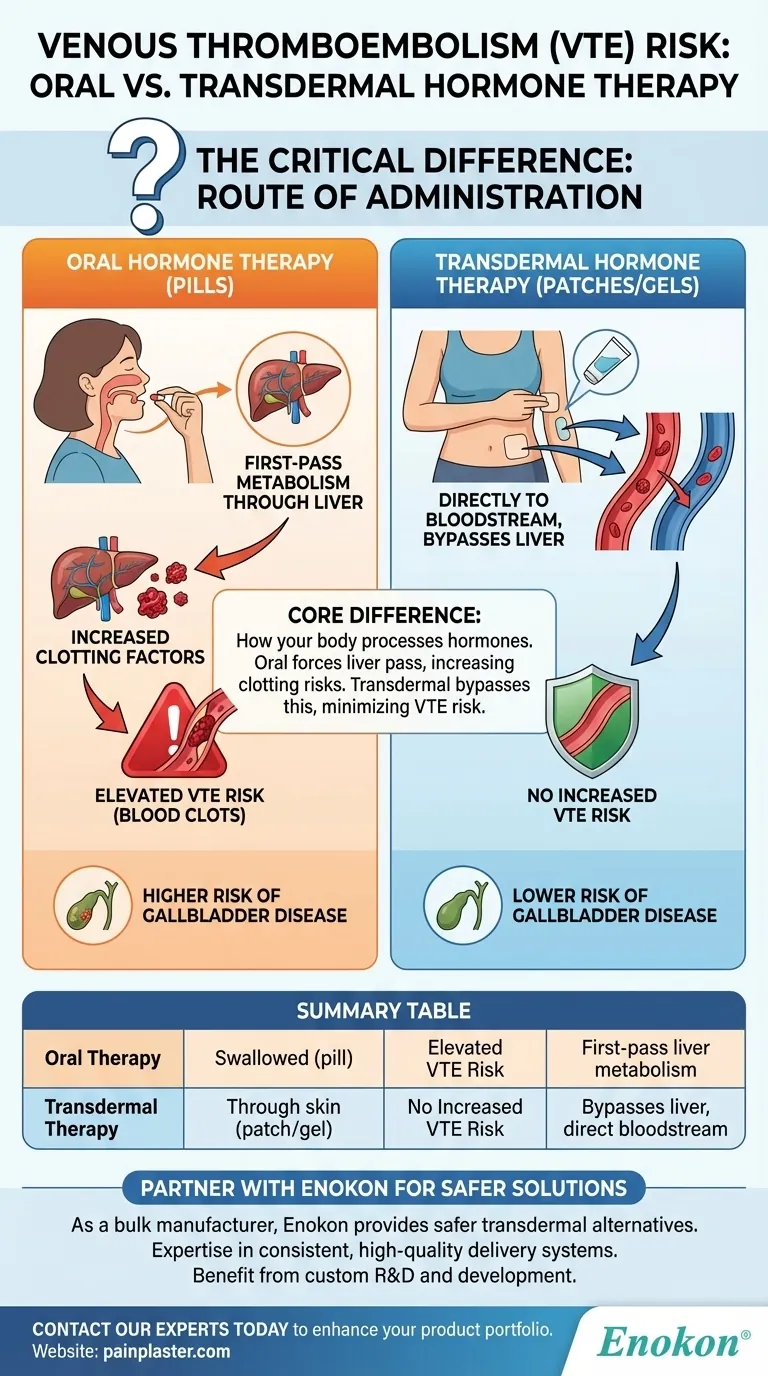
For menopausal hormone therapy, the method of administration is the critical factor in determining your risk of venous thromboembolism (VTE). Oral hormone therapy is associated with an elevated risk of developing blood clots. In contrast, transdermal hormone therapy—delivered through the skin via patches or gels—does not appear to increase this risk.
The fundamental difference lies in how your body processes the hormones. Oral therapy forces estrogen through the liver in a "first-pass" effect that can increase clotting factors, while transdermal therapy delivers it directly to the bloodstream, bypassing this initial liver metabolism.

The Core Difference: Route of Administration
The distinction between taking a pill and using a skin patch might seem small, but it has significant metabolic consequences. This difference is central to understanding the varying safety profiles of hormone therapy options.
How Oral Hormone Therapy Impacts VTE Risk
When you swallow an estrogen pill, it is absorbed through your digestive system and travels directly to the liver.
This process, known as first-pass metabolism, exposes the liver to a high concentration of hormones. In response, the liver can increase its production of proteins involved in blood coagulation, raising the overall risk of forming a clot.
How Transdermal Therapy Avoids This Risk
Transdermal methods, such as patches or gels, deliver hormones directly through the skin into the bloodstream.
This route completely bypasses the first-pass effect in the liver. Because the liver isn't prompted to produce excess clotting factors, transdermal hormone therapy does not carry the same elevated risk of VTE.
Understanding the Associated Health Risks
While VTE is a primary concern, the choice between oral and transdermal therapy can also impact other areas of your health. Understanding these factors provides a more complete picture for decision-making.
The Risk of Venous Thromboembolism (VTE)
VTE is a serious condition where a blood clot forms in a deep vein, most often in the leg (deep vein thrombosis, or DVT). If a piece of that clot breaks off and travels to the lungs, it can cause a life-threatening pulmonary embolism (PE).
It is this specific risk that is elevated with oral hormone preparations but not with transdermal ones.
The Impact on Gallbladder Health
The route of administration also affects gallbladder health. Evidence from the UK Million Women Study highlights this connection.
The study found that the risk of gallbladder disease was significantly lower for women using transdermal therapy compared to those on oral therapy. While both methods showed a slightly higher risk than never using hormone therapy, the oral route posed a greater threat.
Making the Right Choice for Your Health
Choosing the appropriate type of hormone therapy is a critical decision that must be personalized to your unique health profile and risk factors. This discussion should always be had in partnership with your healthcare provider.
- If your primary focus is minimizing VTE and gallbladder risk: Transdermal hormone therapy is the clearly preferred option, as it does not appear to elevate these specific risks.
- If you are considering oral therapy: It is crucial to have a detailed discussion with your doctor about your personal risk factors for blood clots, such as age, weight, smoking status, and family history.
Ultimately, understanding how different delivery methods impact your body empowers you to make a more informed and confident health decision.
Summary Table:
| Hormone Therapy Type | Route of Administration | Impact on VTE Risk | Key Reason |
|---|---|---|---|
| Oral Therapy | Swallowed (pill) | Elevated Risk | First-pass liver metabolism increases clotting factors. |
| Transdermal Therapy | Through skin (patch/gel) | No Increased Risk | Bypasses liver, enters bloodstream directly. |
Partner with Enokon for Safer Hormone Therapy Solutions
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon provides healthcare and pharmaceutical distributors and brands with a safer alternative to oral hormone therapy. Our technical expertise ensures consistent, high-quality transdermal delivery systems that help minimize patient risks like VTE.
Benefit from our custom R&D and development services to create hormone therapy patches tailored to your specific needs.
Contact our experts today to discuss how our transdermal technology can enhance your product portfolio and patient safety.
Visual Guide
Related Products
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Heat Relief Capsicum Patch for Lower Back Pain Relief
People Also Ask
- How can someone switch to HRT patches from another form of HRT? A Guide to a Smooth Transition
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- Can heating pads be used with pain patches? Risks & Safety Tips
- Why should heating pads not be used with transdermal patches? Avoid Overdose & Skin Risks














