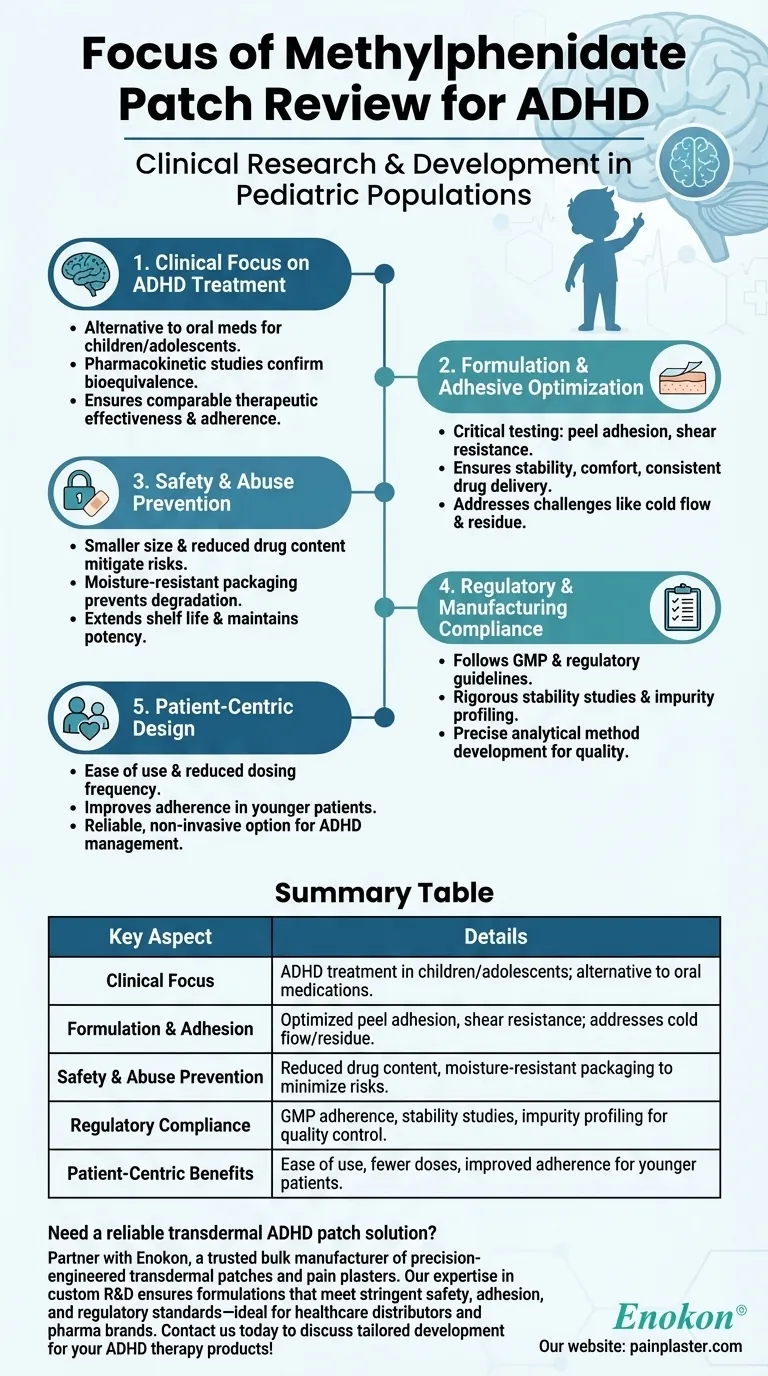
The review primarily examines the clinical research and development of the methylphenidate patch for treating ADHD in children and adolescents. It highlights the patch's formulation, adhesive properties, stability, and safety features, such as reduced drug content and moisture-resistant packaging to minimize risks. The review also covers regulatory compliance, bioequivalence studies, and pharmacokinetic evaluations to ensure efficacy and adherence.

Key Points Explained:
-
Clinical Focus on ADHD Treatment
- The review centers on the methylphenidate patch's application for ADHD in pediatric populations, emphasizing its role as an alternative to oral medications.
- Clinical research includes pharmacokinetic studies to confirm bioequivalence and adherence, ensuring therapeutic effectiveness comparable to traditional forms.
-
Formulation and Adhesive Optimization
- Critical adhesive properties were tested (e.g., peel adhesion, shear resistance) to guarantee patch stability and patient comfort during wear.
- Challenges like cold flow and adhesive residue were addressed to maintain consistent drug delivery and skin compatibility.
-
Safety and Abuse Prevention
- The patch design incorporates a smaller size and lower drug content to mitigate risks of accidental exposure or misuse, a key consideration for stimulant medications.
- Moisture-resistant packaging was developed to prevent chemical degradation, extending shelf life and maintaining potency.
-
Regulatory and Manufacturing Compliance
- Development followed Good Manufacturing Practices (GMP) and regulatory guidelines, with rigorous stability studies and impurity profiling to ensure quality control.
- Analytical method development supported precise measurement of drug release and impurity levels, aligning with safety standards.
-
Patient-Centric Design
- The review underscores practical advantages, such as ease of use and reduced dosing frequency, which may improve adherence in younger patients.
- By addressing formulation challenges, the patch aims to offer a reliable, non-invasive option for ADHD management.
This comprehensive analysis bridges clinical needs with technical innovation, offering insights into how the methylphenidate patch could reshape ADHD therapy.
Summary Table:
| Key Aspect | Details |
|---|---|
| Clinical Focus | ADHD treatment in children/adolescents; alternative to oral medications. |
| Formulation & Adhesion | Optimized peel adhesion, shear resistance; addresses cold flow/residue. |
| Safety & Abuse Prevention | Reduced drug content, moisture-resistant packaging to minimize risks. |
| Regulatory Compliance | GMP adherence, stability studies, impurity profiling for quality control. |
| Patient-Centric Benefits | Ease of use, fewer doses, improved adherence for younger patients. |
Need a reliable transdermal ADHD patch solution? Partner with Enokon, a trusted bulk manufacturer of precision-engineered transdermal patches and pain plasters. Our expertise in custom R&D ensures formulations that meet stringent safety, adhesion, and regulatory standards—ideal for healthcare distributors and pharma brands. Contact us today to discuss tailored development for your ADHD therapy products!
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Capsaicin Chili Medicated Pain Relief Patches
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- How do pain relief patches provide targeted relief? Discover the Science Behind Effective Pain Management
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief
- How effective are pain relief patches for muscle pain? Target Localized Pain with Transdermal Delivery
- How do pain relief patches compare to other pain relief methods? Discover Targeted, Long-Lasting Relief














