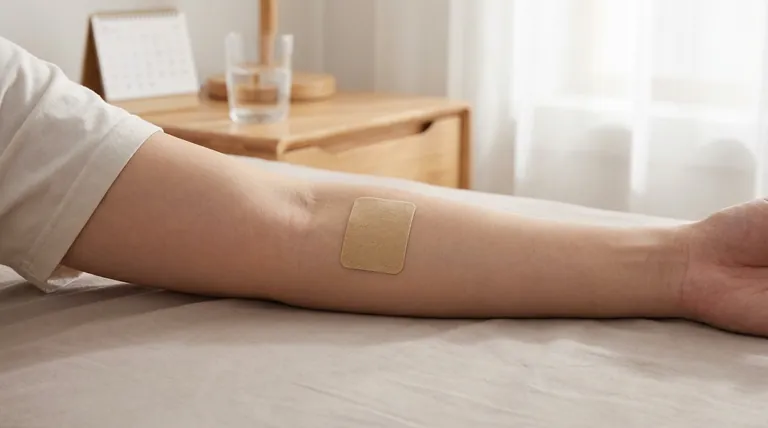
The generic name for the clonidine transdermal system is simply clonidine. It is supplied as an extended-release transdermal film, which is a patch applied directly to the skin. The available dosage strengths are designed to deliver 0.1 mg, 0.2 mg, or 0.3 mg of the medication per 24-hour period.
The key function of the clonidine transdermal patch is to provide a steady, continuous release of medication over an entire week. This delivery system is designed to maintain stable drug levels in the blood, avoiding the peaks and troughs associated with oral tablets.
How the Transdermal System Works
The clonidine patch is more than just a sticker with medicine; it's an engineered drug delivery system. Understanding its mechanics is crucial for appreciating its clinical use.
The Concept of Extended-Release
Each patch is designed to be worn for seven consecutive days. Throughout this period, it slowly and consistently releases clonidine through the skin and into the bloodstream.
This steady-state delivery is the primary advantage of the transdermal route for this medication.
Dosage vs. Delivery Rate
The dosage strengths—0.1 mg/24 hr, 0.2 mg/24 hr, and 0.3 mg/24 hr—refer to the rate of delivery. This is the amount of drug the patient absorbs each day.
It is important to note that the total amount of clonidine in the patch is higher than the daily dose. This surplus ensures the patch can maintain its specified delivery rate for the full seven-day period.
The Rationale for a Transdermal Patch
Choosing a patch over a pill is a deliberate clinical decision based on specific therapeutic goals. The transdermal system offers distinct advantages in managing chronic conditions like hypertension.
Improving Medication Adherence
A once-weekly application is often much easier for patients to manage than remembering to take pills multiple times a day. This simplicity can significantly improve adherence to the treatment plan.
Ensuring Stable Blood Levels
Oral medications can cause sharp spikes and dips in blood concentration. The transdermal patch smooths these fluctuations out, leading to more consistent blood pressure control and potentially fewer side effects related to high peak drug levels.
Bypassing the Digestive System
By absorbing the medication through the skin, the drug enters the bloodstream directly. This avoids the "first-pass effect," where the liver metabolizes a portion of the drug before it can circulate through the body, which can be an advantage for certain medications.
Understanding the Trade-offs
While effective, the transdermal system is not without its own set of challenges. Objectivity requires acknowledging its potential downsides.
Potential for Skin Irritation
The most common side effect is localized skin reaction at the application site. This can include redness, itching, or a rash. Rotating the application site each week is critical to minimize this risk.
Slower Onset of Action
Unlike an oral tablet that acts relatively quickly, a transdermal patch takes time to deliver enough medication to reach a therapeutic level. It is not suitable for situations requiring rapid blood pressure reduction.
Adhesion Issues
The patch must remain in full contact with the skin to work correctly. Factors like excessive sweating, swimming, or improper application can cause the patch to loosen or fall off, interrupting drug delivery.
Making the Right Choice for Your Goal
Selecting the appropriate dosage form depends entirely on the clinical objective and patient profile.
- If your primary focus is consistent blood pressure control: The transdermal patch excels at providing stable drug levels, minimizing the fluctuations seen with oral tablets.
- If your primary concern is ease of use and medication adherence: The once-weekly application simplifies the treatment regimen significantly compared to multiple daily doses.
- If you have sensitive skin or a history of skin reactions: Be aware that local skin irritation is a common side effect that may require careful monitoring or an alternative form of medication.
Understanding the specific delivery mechanism of the clonidine patch is key to using it safely and effectively.
Summary Table:
| Aspect | Details |
|---|---|
| Generic Name | Clonidine |
| Dosage Form | Extended-release transdermal film (patch) |
| Available Strengths | 0.1 mg/24 hr, 0.2 mg/24 hr, 0.3 mg/24 hr |
| Application Frequency | Once weekly (7-day wear) |
| Primary Advantage | Steady, continuous drug delivery for stable blood levels |
Need a reliable transdermal patch solution?
As Enokon, a bulk manufacturer of high-quality transdermal patches and pain plasters, we specialize in custom R&D and development for healthcare and pharma distributors and brands. Our technical expertise ensures you get a product tailored to your specific needs, from precise dosage control to optimal adhesion.
Contact our experts today to discuss your custom transdermal project.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Herbal Eye Protection Patch Eye Patch
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
















