In short, the lidocaine 5% patch is a topical pain reliever officially approved by the FDA to treat the nerve pain that can follow a shingles infection, a condition known as post-herpetic neuralgia (PHN). It works by delivering a local anesthetic directly to the painful area of the skin, effectively numbing the superficial nerves and providing relief without significant systemic side effects.
The core principle of the lidocaine 5% patch is targeted relief. While its only official FDA approval is for post-shingles pain, its ability to numb a specific area with minimal absorption into the bloodstream has led to its widespread use for various other types of localized chronic pain.
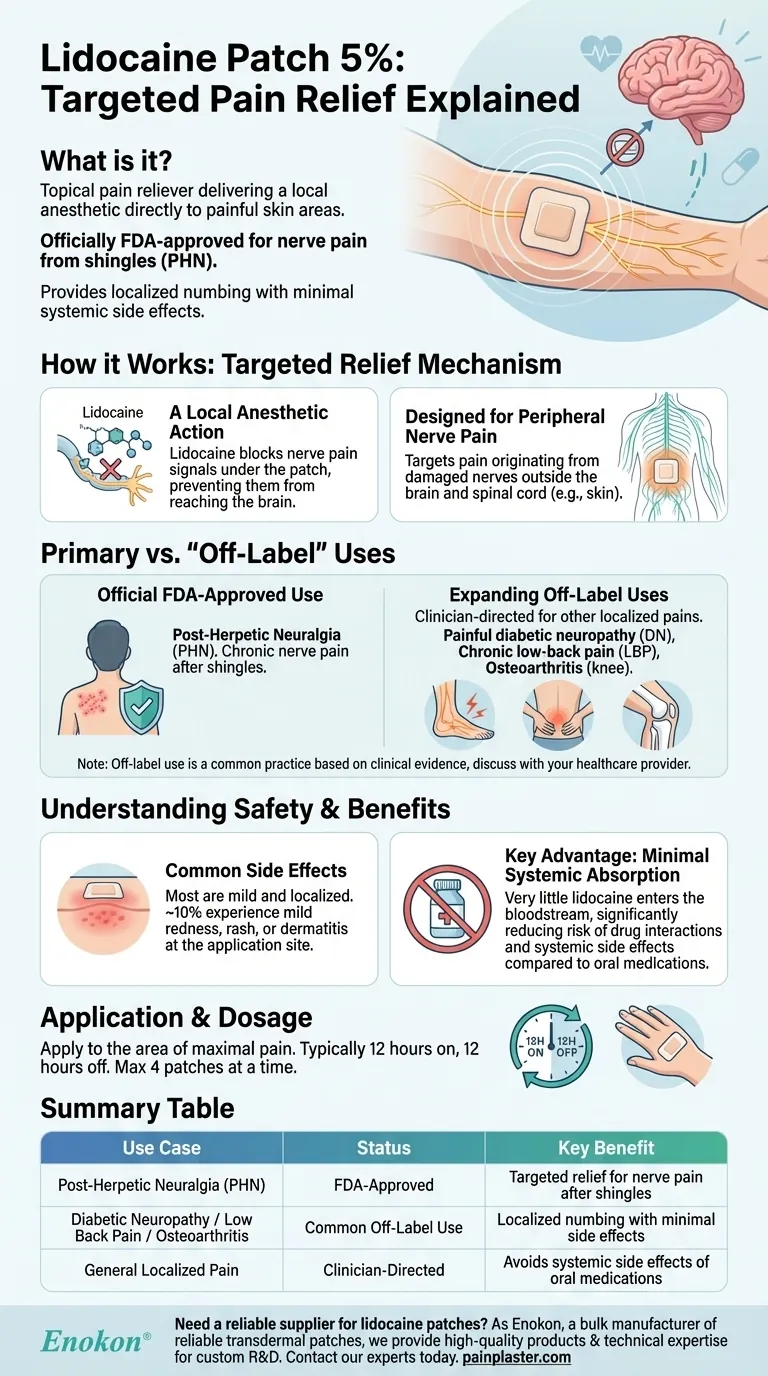
How the Lidocaine Patch Provides Targeted Relief
A Local Anesthetic Action
The lidocaine patch 5% is a topical analgesic, meaning it's a pain reliever applied directly to the skin.
Its active ingredient, lidocaine, blocks the pain signals in the nerves right under the patch. This prevents those signals from reaching your brain, providing relief in a very specific, localized area.
Designed for Peripheral Nerve Pain
The patch was specifically developed to treat peripherally generated neuropathic pain.
This is pain that originates from damaged nerves located away from the central nervous system (the brain and spinal cord), such as in the skin.
Primary vs. "Off-Label" Uses
The Official FDA-Approved Use: Post-Herpetic Neuralgia (PHN)
The single, officially approved indication for the lidocaine 5% patch in the United States is for the pain of post-herpetic neuralgia (PHN).
PHN is a painful, chronic condition that can occur after a shingles outbreak, causing burning, stabbing, or aching nerve pain in the area of the former rash.
Expanding Use for Other Localized Pains
Due to its effectiveness and safety profile, clinicians often use the patch for other conditions where pain is localized. Studies have explored its impact on:
- Painful diabetic neuropathy (DN)
- Chronic low-back pain (LBP)
- Pain from osteoarthritis, particularly in the knee
It's important to understand that using the patch for these conditions is considered "off-label," but it is a common practice based on clinical evidence of its utility.
Understanding the Trade-offs and Safety Profile
Common Side Effects
The vast majority of side effects are mild, localized, and related to the application site.
About 10% of patients may experience mild-to-moderate reactions like skin redness, rash, or dermatitis where the patch is applied. Less common effects can include headache or a taste disturbance.
The Key Advantage: Minimal Systemic Absorption
A major benefit of the patch is its minimal systemic toxicity. Very little of the lidocaine is absorbed into the bloodstream.
This dramatically reduces the risk of the drug interactions and systemic side effects often seen with oral pain medications, making it a well-tolerated option for many people.
Application and Dosage
Patients typically apply one or more patches to the area of maximal pain for a set period each day, usually 12 hours on and 12 hours off.
The maximum recommended dose is typically no more than four patches used at one time.
Making the Right Choice for Your Goal
When considering the lidocaine patch, it's essential to align its properties with your specific pain management needs.
- If your primary focus is treating nerve pain after a shingles infection: The lidocaine 5% patch is the specific, FDA-approved treatment for this condition.
- If your primary focus is managing other localized pains (like knee or low back): The patch is a well-tolerated option that may provide significant relief, but this use should be discussed with your healthcare provider.
- If your primary focus is avoiding the side effects of oral pain pills: The patch's localized action and minimal absorption into the body make it a strong alternative for targeted pain.
Ultimately, the lidocaine 5% patch is a valuable tool for managing pain that is confined to a specific area of your body.
Summary Table:
| Use Case | Status | Key Benefit |
|---|---|---|
| Post-Herpetic Neuralgia (PHN) | FDA-Approved | Targeted relief for nerve pain after shingles |
| Diabetic Neuropathy / Low Back Pain / Osteoarthritis | Common Off-Label Use | Localized numbing with minimal side effects |
| General Localized Pain | Clinician-Directed | Avoids systemic side effects of oral medications |
Need a reliable supplier for lidocaine patches?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we provide healthcare and pharma distributors and brands with high-quality, consistent products. Benefit from our technical expertise for custom R&D and development to create the pain management solutions your customers need.
Contact our experts today to discuss your requirements.
Visual Guide
Related Products
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- What are the benefits of using a pain relief patch instead of oral medication? Get Targeted, Long-Lasting Relief
- How long can Pain Relief Patch be worn? A Guide to the 12-Hour Rule for Safe Relief
- What are the active ingredients in the pain relief patch? Unlocking the Dual-Action Formula
- What are the benefits of Pain Relief Patch being licensed as a medicine? Guaranteed Efficacy & Safety
- Can children use the pain relief patch? A Critical Safety Guide for Parents

















