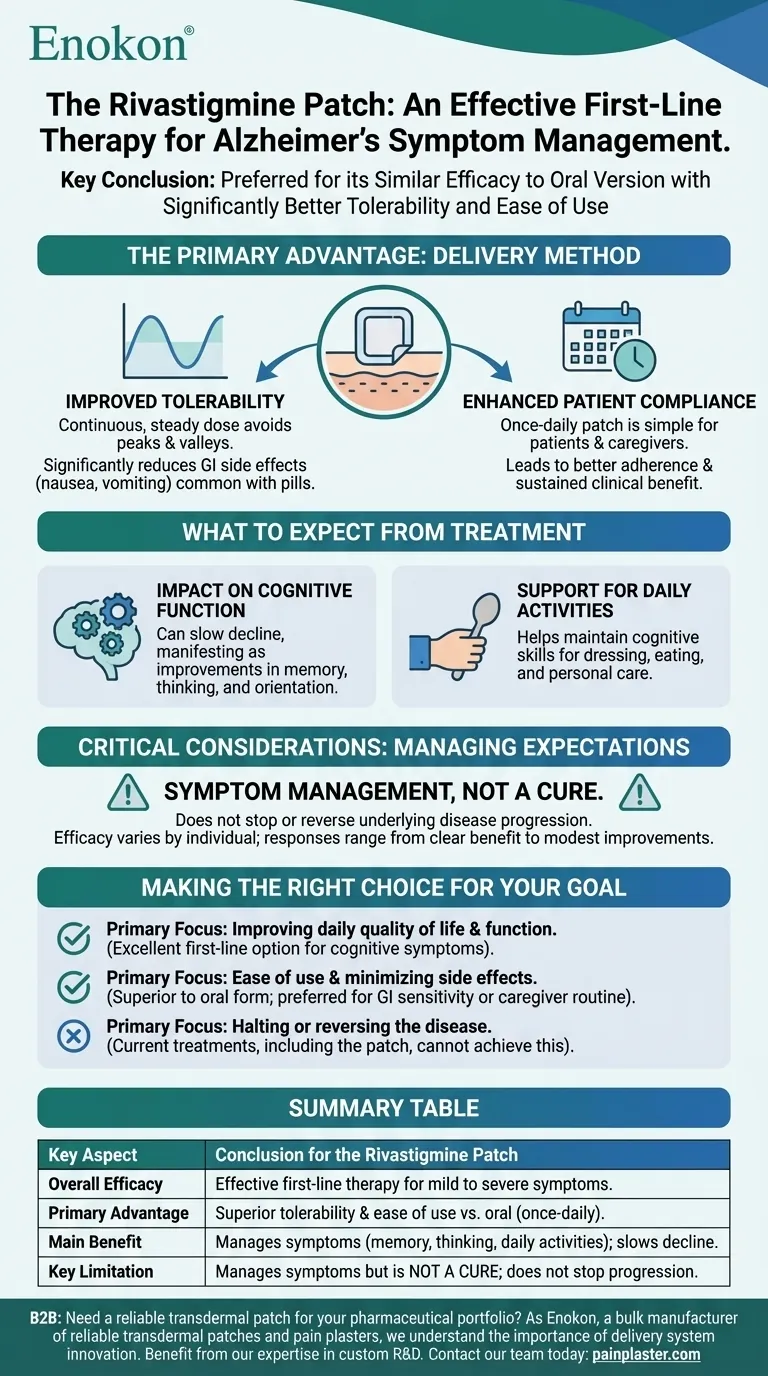
The definitive conclusion is that the rivastigmine patch is an effective and often preferred first-line therapy for managing the symptoms of mild to severe Alzheimer's disease. Its primary advantage is not that it offers a more powerful treatment, but that it provides a similar therapeutic benefit to the oral version with significantly better tolerability and ease of use. This makes it a valuable tool for improving a patient's daily life and easing the burden on caregivers.
The rivastigmine patch should be viewed as a tool for symptom management, not a cure. Its core value lies in its delivery system, which improves quality of life by reducing side effects and simplifying the treatment regimen, but it does not stop or reverse the underlying progression of the disease.
The Primary Advantage: Delivery Method
The key innovation of the rivastigmine patch is not the drug itself, but how it is administered. This change in delivery directly addresses the most common challenges associated with the oral version of the medication.
Improved Tolerability
By delivering a continuous, steady dose of rivastigmine through the skin, the patch avoids the peaks and valleys in medication levels that occur with pills. This consistent delivery significantly reduces the gastrointestinal side effects, like nausea and vomiting, that are common with the oral form.
Enhanced Patient Compliance
A once-daily patch is far simpler for both patients and caregivers to manage than multiple daily pills. This ease of use leads to better adherence to the treatment plan, ensuring the patient receives a sustained clinical benefit.
What to Expect from Treatment
It is crucial to have realistic expectations about what the rivastigmine patch can and cannot do. Its benefits are focused on managing symptoms and maintaining function for as long as possible.
Impact on Cognitive Function
For many patients, the patch can help slow the decline of cognitive functions. This may manifest as improvements in memory, thinking, and the ability to orient oneself.
Support for Daily Activities
A key therapeutic goal is to help patients maintain their ability to perform daily activities. The patch can support this by improving the cognitive skills necessary for tasks like dressing, eating, and personal care.
Critical Considerations: Managing Expectations
Understanding the limitations of the rivastigmine patch is as important as understanding its benefits. This is not a miracle drug, but a supportive therapy.
This is Symptom Management, Not a Cure
The most important point to understand is that the rivastigmine patch does not cure Alzheimer's disease. It does not alter the progressive course of the underlying illness. Its function is to manage symptoms and potentially slow the rate of functional decline.
Efficacy Varies by Individual
As with any medication for dementia, the response to the rivastigmine patch can vary significantly from one person to another. While many experience a clear benefit, others may see only modest improvements or none at all.
Making the Right Choice for Your Goal
When considering this treatment, align your expectations with the specific goal you hope to achieve.
- If your primary focus is improving daily quality of life and function: The patch is an excellent first-line option due to its proven efficacy in managing cognitive symptoms.
- If your primary focus is ease of use and minimizing side effects: The patch is clearly superior to the oral form, making it the preferred choice for patients sensitive to gastrointestinal issues or for simplifying a caregiver's routine.
- If your primary focus is halting or reversing the disease: The rivastigmine patch, like all current Alzheimer's treatments, cannot achieve this goal.
Ultimately, the rivastigmine patch serves as a valuable tool for managing symptoms and improving the daily lives of those affected by Alzheimer's disease.
Summary Table:
| Key Aspect | Conclusion for the Rivastigmine Patch |
|---|---|
| Overall Efficacy | Effective first-line therapy for mild to severe Alzheimer's symptoms. |
| Primary Advantage | Superior tolerability & ease of use vs. oral version (once-daily patch). |
| Main Benefit | Manages symptoms (memory, thinking, daily activities); slows decline. |
| Key Limitation | Manages symptoms but is not a cure; does not stop disease progression. |
Need a reliable transdermal patch for your pharmaceutical portfolio?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we understand the critical importance of delivery system innovation for patient quality of life. Our technical expertise in custom R&D and development can help you bring effective, user-friendly treatments to market.
Benefit from our expertise to develop your next transdermal solution. Contact our team today to discuss your needs.
Visual Guide
Related Products
- Herbal Eye Protection Patch Eye Patch
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- How quickly can you see results from using under eye patches? Instant Brightening & Long-Term Benefits
- What are the main benefits of using eye patches in a skincare routine? Revitalize Your Under-Eye Area
- How can using eye patches contribute to a self-care skincare routine? Boost Hydration & Relaxation
- What are the steps for properly using eye patches? Maximize Benefits for Your Delicate Eye Area
- What factors should be considered when purchasing eye patches? Essential Guide for Safe & Effective Use














