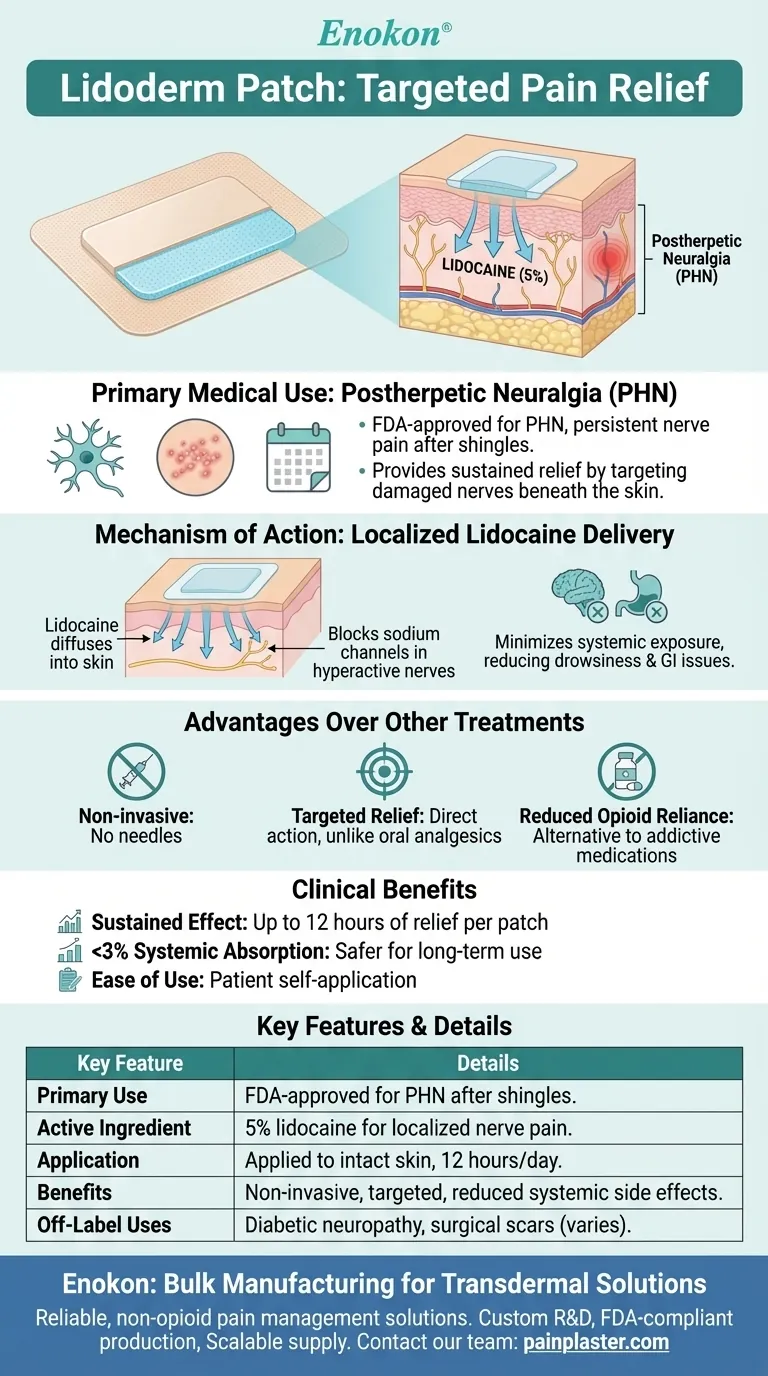
The Lidoderm patch is a medicated adhesive patch containing lidocaine, a local anesthetic, designed to provide targeted pain relief. Its primary medical use is for managing postherpetic neuralgia (PHN), a persistent nerve pain condition that can occur after a shingles outbreak. The patch works by releasing lidocaine directly to the affected skin area, blocking pain signals without significant systemic absorption. This localized approach minimizes side effects while offering an alternative to oral pain medications, including opioids. Lidoderm patches represent a non-invasive, convenient option for patients suffering from PHN, addressing neuropathic pain at its source.
Key Points Explained:
-
Primary Medical Use: Postherpetic Neuralgia (PHN)
- The Lidoderm patch is FDA-approved specifically for PHN, a complication of shingles (herpes zoster) where nerve pain persists after the rash heals.
- PHN can last months or years, and the patch provides sustained relief by targeting the damaged nerves beneath the skin.
-
Mechanism of Action: Localized Lidocaine Delivery
- The patch contains 5% lidocaine, which diffuses into the skin to block sodium channels in hyperactive nerves, preventing pain signal transmission.
- Unlike oral medications, the patch minimizes systemic exposure, reducing risks like drowsiness or gastrointestinal issues.
-
Advantages Over Other Treatments
- Non-invasive: No needles or injections required, ideal for patients averse to invasive procedures.
- Targeted relief: Acts directly on the painful area, unlike oral analgesics that affect the entire body.
- Reduced opioid reliance: Offers an alternative for patients seeking to avoid opioids or NSAIDs, which carry addiction and side-effect risks.
-
Clinical Benefits
- Sustained effect: Each patch provides up to 12 hours of pain relief per application.
- Low systemic absorption: Less than 3% of the lidocaine enters the bloodstream, making it safer for long-term use.
- Ease of use: Patients can apply it themselves to intact skin, following medical guidance.
-
Considerations for Use
- Prescription-only: Requires a doctor’s supervision to ensure proper diagnosis (e.g., ruling out non-PHN pain).
- Application guidelines: Typically applied to intact skin (avoiding open wounds) for 12 hours/day, with dosage adjustments for sensitive patients.
- Side effects: Mild skin reactions (redness, rash) may occur but are less common than with systemic treatments.
-
Expanding Applications
- While PHN is the primary use, some clinicians prescribe Lidoderm off-label for other localized neuropathic pain (e.g., diabetic neuropathy or surgical scars), though efficacy varies.
By focusing on PHN, the Lidoderm patch addresses a critical gap in pain management—providing a safer, localized solution for a condition that significantly impacts quality of life. Its design exemplifies how targeted drug delivery can optimize therapeutic outcomes while minimizing risks.
Summary Table:
| Key Feature | Details |
|---|---|
| Primary Use | FDA-approved for postherpetic neuralgia (PHN) after shingles. |
| Active Ingredient | 5% lidocaine for localized nerve pain blocking. |
| Application | Applied to intact skin for up to 12 hours/day. |
| Benefits | Non-invasive, targeted relief, reduced systemic side effects vs. oral meds. |
| Off-Label Uses | Diabetic neuropathy, surgical scars (varies by patient). |
Need a reliable, non-opioid pain management solution?
Enokon specializes in bulk manufacturing of high-quality transdermal patches, including lidocaine-based formulations like the Lidoderm patch. Our expertise ensures:
- Custom R&D for tailored pain relief solutions.
- FDA-compliant production for healthcare distributors and brands.
- Scalable supply with rigorous quality control.
Let’s discuss how we can support your PHN or neuropathic pain product line—contact our team today!
Visual Guide
Related Products
- Herbal Eye Protection Patch Eye Patch
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Hydra Gel Health Care Eye Patch
- Icy Hot Menthol Medicine Pain Relief Patch
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- What are the steps for applying under-eye patches? Boost Your Eye Care Routine
- What factors should be considered when purchasing eye patches? Essential Guide for Safe & Effective Use
- How quickly can you see results from using under eye patches? Instant Brightening & Long-Term Benefits
- Should under eye patches be applied before or after moisturizer? Optimize Your Skincare Routine
- What are the steps for properly using eye patches? Maximize Benefits for Your Delicate Eye Area














