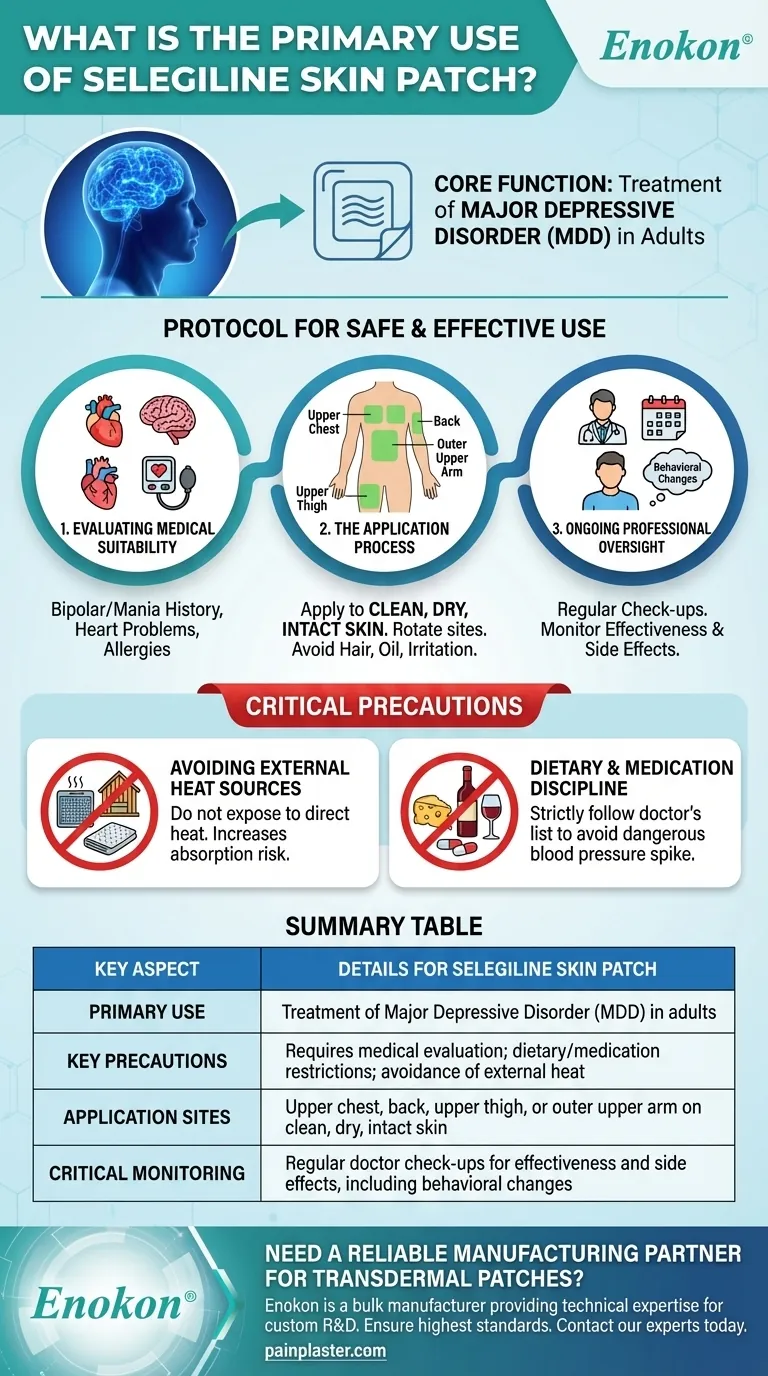
The selegiline skin patch is a transdermal system prescribed for a very specific and serious medical condition. Its primary and approved use is for the treatment of major depressive disorder, also known as mental depression, in adult patients.
While its purpose is to treat depression, the safety and effectiveness of the selegiline patch are critically dependent on a comprehensive medical evaluation, strict adherence to application protocols, and a commitment to specific lifestyle modifications.

The Core Function: Treating Depression
The selegiline patch is a targeted therapy that requires a clear understanding of its intended use and the patient's overall health profile before treatment begins.
Evaluating Medical Suitability
Before prescribing this medication, a healthcare provider must weigh its potential benefits against the risks. A thorough evaluation is essential.
Several pre-existing medical conditions can significantly impact the safety of using the selegiline patch, including a history of bipolar disorder, mania, heart problems, low blood pressure, or pheochromocytoma (a type of adrenal gland tumor).
Other critical factors include known allergies, age-related considerations for geriatric patients, and whether the patient is breastfeeding.
Protocol for Safe and Effective Use
Proper administration and ongoing management are not just recommendations; they are essential for the medication to work safely.
The Application Process
The patch must be applied to clean, dry, and intact skin.
Approved application sites are the upper chest, back, upper thigh, or the outer part of the upper arm. It is vital to choose a new spot for each application.
Avoid applying the patch to areas with hair, oil, irritation, scars, or where tight clothing may rub against it. After applying, press down firmly to ensure it is fully adhered.
Ongoing Professional Oversight
Regular check-ups with your doctor are a mandatory part of this treatment. These appointments are used to monitor the medication's effectiveness and manage any side effects.
A crucial aspect of monitoring is watching for any significant behavioral changes or the emergence of suicidal thoughts, which requires immediate medical attention.
Understanding the Critical Precautions
Using the selegiline patch involves a partnership between the patient and their doctor, with a shared responsibility for managing potential risks.
Avoiding External Heat Sources
The patch should never be exposed to direct heat sources like heating pads, electric blankets, heat lamps, or saunas. Excessive heat can increase the amount of medication absorbed by the body, leading to dangerous side effects.
Critical Dietary and Medication Discipline
Patients must avoid certain foods, beverages, and other medicines while using the selegiline patch. Your doctor and pharmacist will provide a specific list, which often includes aged cheeses, certain meats, and alcoholic beverages.
Failure to follow these dietary restrictions can cause a sudden, severe increase in blood pressure, which is a medical emergency.
Making an Informed Decision
Successfully using the selegiline patch is contingent on a clear understanding of its requirements and a strong commitment to following medical guidance.
- If you are considering this treatment for depression: Your primary step is a transparent conversation with your doctor about your complete medical history to determine if the benefits outweigh the significant risks.
- If you have been prescribed the selegiline patch: Strict adherence to application instructions, dietary restrictions, and regular medical monitoring is non-negotiable for your safety.
Understanding these responsibilities is the foundation for using this specialized treatment safely and effectively.
Summary Table:
| Key Aspect | Details for the Selegiline Skin Patch |
|---|---|
| Primary Use | Treatment of Major Depressive Disorder (MDD) in adults |
| Key Precautions | Requires medical evaluation; dietary/medication restrictions; avoidance of external heat |
| Application Sites | Upper chest, back, upper thigh, or outer upper arm on clean, dry, intact skin |
| Critical Monitoring | Regular doctor check-ups for effectiveness and side effects, including behavioral changes |
Need a reliable manufacturing partner for transdermal patches like selegiline?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we provide healthcare and pharma distributors and brands with the technical expertise for custom R&D and development. Ensure your products meet the highest standards of safety and efficacy.
Contact our experts today to discuss your specific transdermal patch needs.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Far Infrared Pain Patch Relief Pain Reliever for Back
- Menthol Gel Pain Relief Patch
People Also Ask
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief
- What are pain relief patches and how are they used? A Guide to Safe, Targeted Relief
- How effective are pain relief patches for muscle pain? Target Localized Pain with Transdermal Delivery

















