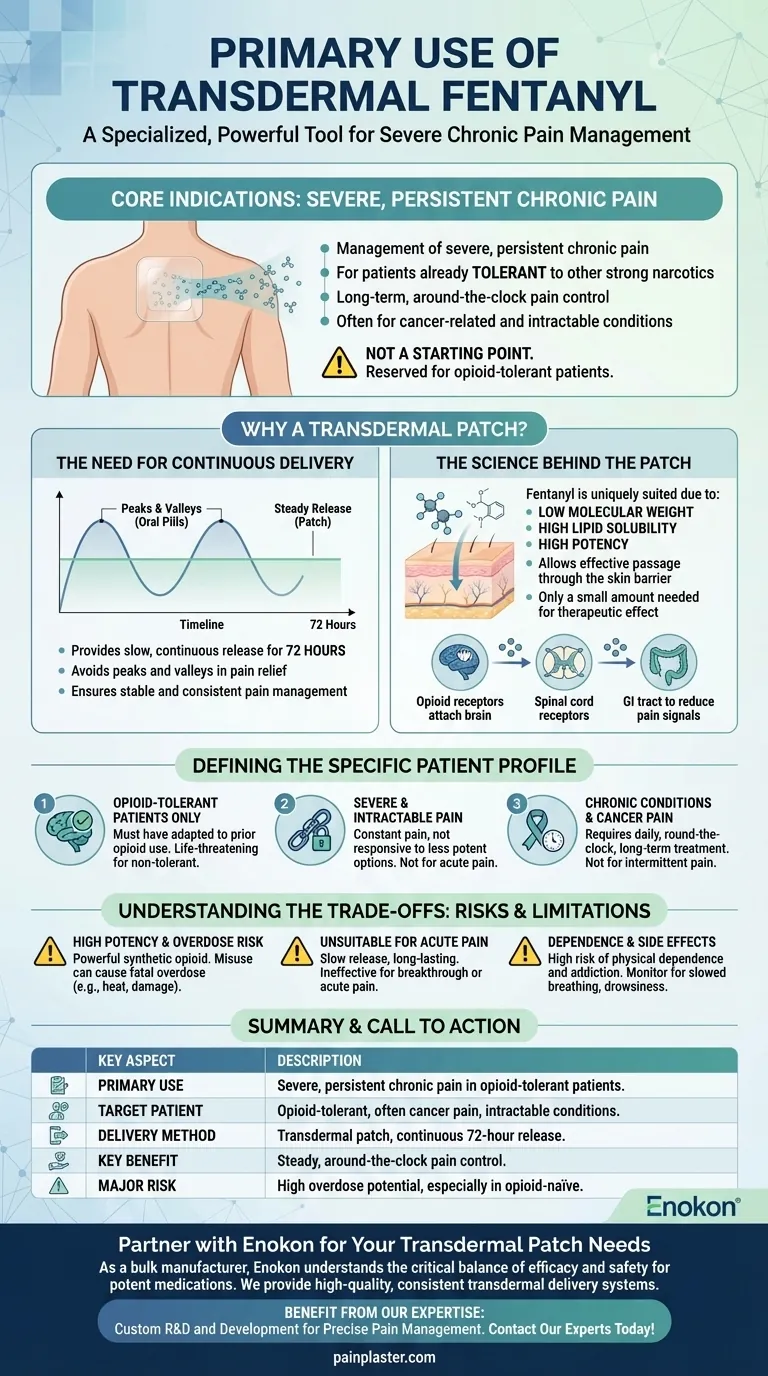
The primary use of transdermal fentanyl is for the management of severe, persistent chronic pain in patients who are already tolerant to other strong narcotic medications. It is specifically designed for long-term, around-the-clock pain control where other treatment options have proven insufficient, most commonly for cancer-related pain and other intractable conditions.
Transdermal fentanyl is not a starting point for pain relief. It is a specialized, powerful tool reserved for opioid-tolerant individuals who require continuous, steady delivery of a potent analgesic to manage severe, chronic pain that is otherwise unmanageable.

Why a Transdermal Patch is Used
The choice of a skin patch for a drug as potent as fentanyl is highly intentional, designed to solve specific challenges in managing chronic pain.
The Need for Continuous Delivery
Severe chronic pain is often constant, not intermittent. A transdermal patch provides a slow, continuous release of fentanyl into the bloodstream over a 72-hour period.
This steady delivery avoids the peaks and valleys in pain relief and medication levels that can occur with oral pills, leading to more stable and consistent pain management.
The Science Behind the Patch
Fentanyl is uniquely suited for transdermal delivery. Its chemical properties, including a low molecular weight and high lipid solubility, allow it to pass through the skin barrier effectively.
Combined with its high potency, only a small amount is needed to achieve a therapeutic effect, making a small, wearable patch a viable delivery system.
How It Works in the Body
Once in the bloodstream, fentanyl works by attaching to opioid receptors located in the brain, spinal cord, and gastrointestinal tract.
By binding to these receptors, it effectively reduces the pain signals sent to the brain, changing how the brain and nervous system respond to and perceive pain.
Defining the Specific Patient Profile
Transdermal fentanyl is FDA-approved only for a very specific type of patient due to its strength and risk profile.
Opioid-Tolerant Patients Only
This is the most critical requirement. The patient must be opioid-tolerant, meaning their body has already adapted to the effects of narcotic pain medications from previous, long-term use.
Prescribing this medication to someone who is not opioid-tolerant can cause a life-threatening overdose, primarily through severe respiratory depression.
Severe and Intractable Pain
The system is intended for pain that is both severe and intractable. This is not for managing headaches, post-surgical pain, or pain that comes and goes.
It is reserved for conditions where the pain is constant and has not responded adequately to less potent opioids or other forms of treatment.
Chronic Conditions and Cancer Pain
The most common applications are for managing the severe, persistent pain associated with cancer.
It is also approved for select cases of chronic non-cancer pain, but only when a patient requires daily, round-the-clock, long-term opioid treatment and other options are unsuitable.
Understanding the Trade-offs
While effective for its intended purpose, transdermal fentanyl carries significant risks and limitations that must be carefully managed.
High Potency and Overdose Risk
Fentanyl is a powerful synthetic opioid. Incorrect use, such as applying heat to the patch, using a damaged patch, or applying more than one at a time, can accelerate drug release and cause a fatal overdose.
Unsuitability for Acute Pain
The slow release and long-lasting effect make the patch completely inappropriate for acute or breakthrough pain. It takes many hours to reach a therapeutic level in the blood and is designed for baseline pain, not immediate relief.
Dependence and Side Effects
Like all strong opioids, transdermal fentanyl carries a high risk of physical dependence and addiction. Patients must be monitored for side effects, which can include drowsiness, confusion, nausea, and dangerously slowed breathing.
How to Apply This to Pain Management
The decision to use this medication is a serious one, based entirely on the specific nature and duration of the pain.
- If your primary focus is managing severe, constant, and long-term pain: Transdermal fentanyl may be a suitable option, but only for patients confirmed to be opioid-tolerant and under strict medical supervision.
- If your primary focus is treating acute or intermittent pain: This medication is inappropriate and dangerous due to its slow onset and long duration of action.
- If a patient is new to opioid medications: Transdermal fentanyl must be strictly avoided, as its high potency can be fatal for individuals who have not developed a tolerance.
Ultimately, transdermal fentanyl is a targeted therapy that offers profound relief for a specific patient population while demanding the utmost respect for its risks.
Summary Table:
| Key Aspect | Description |
|---|---|
| Primary Use | Management of severe, persistent chronic pain in opioid-tolerant patients. |
| Target Patient | Patients already tolerant to strong opioids, often with cancer pain or other intractable conditions. |
| Delivery Method | Transdermal patch providing continuous release over 72 hours. |
| Key Benefit | Steady, around-the-clock pain control, avoiding peaks and valleys of oral medication. |
| Major Risk | High potential for life-threatening overdose, especially in opioid-naïve individuals. |
Partner with Enokon for Your Transdermal Patch Needs
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon understands the critical balance of efficacy and safety required for potent medications like fentanyl. We provide healthcare and pharmaceutical distributors and brands with high-quality, consistent transdermal delivery systems.
Benefit from our technical expertise for custom R&D and development to create the precise pain management solutions your patients need.
Contact our experts today to discuss how we can support your product development with our manufacturing capabilities and technical knowledge.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Icy Hot Menthol Medicine Pain Relief Patch
- Menthol Gel Pain Relief Patch
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Heat Pain Relief Patches Transdermal Patches
People Also Ask
- How effective are pain relief patches for muscle pain? Target Localized Pain with Transdermal Delivery
- How should pain relief patches be applied and used? A Guide to Safe & Effective Targeted Relief
- How do pain relief patches compare to other pain relief methods? Discover Targeted, Long-Lasting Relief
- What are pain relief patches and how are they used? A Guide to Safe, Targeted Relief
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief















