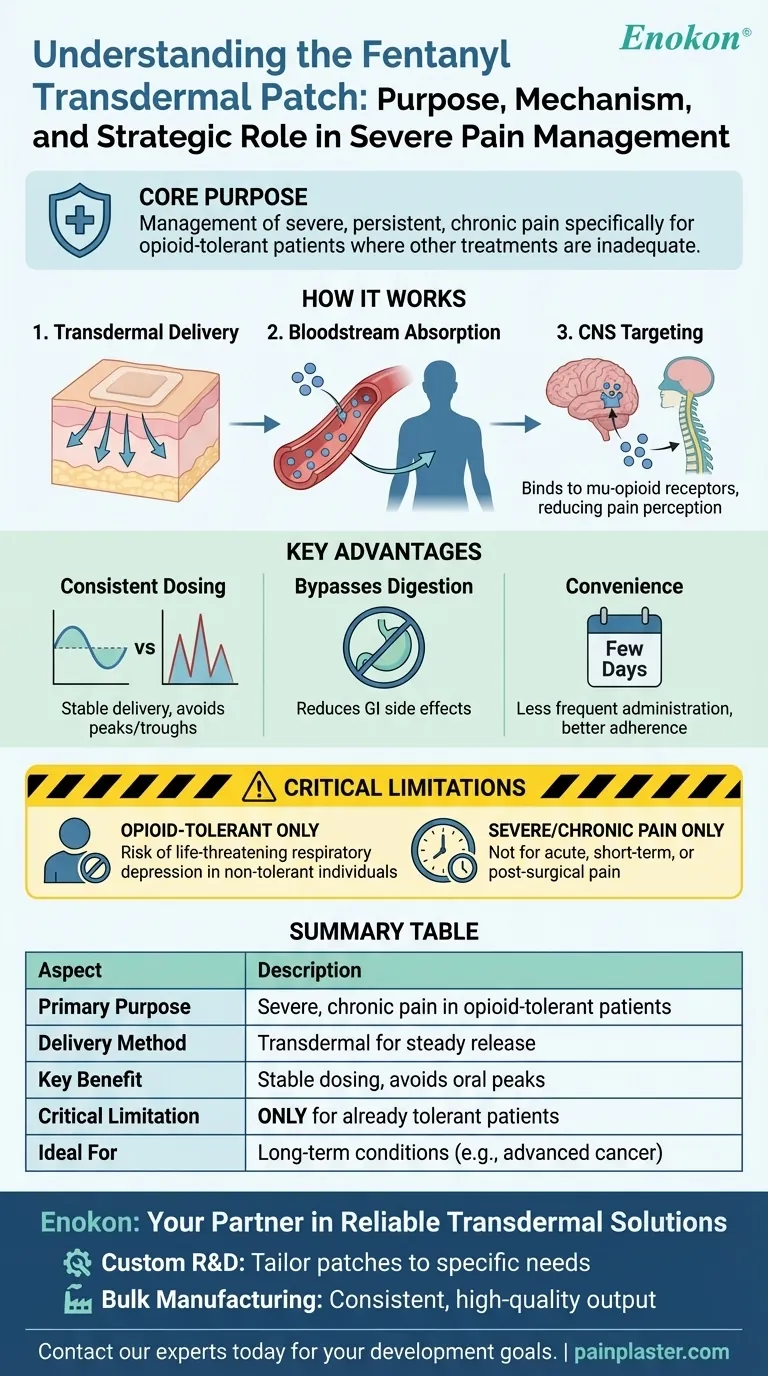
The fentanyl transdermal patch is a powerful medical tool prescribed for the management of severe, persistent pain. Its purpose is to provide continuous, long-term relief specifically for individuals who are already tolerant to other narcotic pain medications and for whom other treatment options are inadequate. It works by altering how the brain and central nervous system perceive pain signals.
The core purpose of the fentanyl patch is not to treat initial or short-term pain, but to deliver a consistent, stable dose of a potent opioid for managing severe, chronic pain in a specific patient population. It leverages the benefits of a patch to provide steady relief that oral medications cannot easily replicate.
How the Fentanyl Patch Works
The Principle of Transdermal Delivery
A transdermal patch is a medicated adhesive that sticks to the skin. This system allows a medication to be absorbed slowly and directly into the bloodstream over an extended period.
This delivery method is not unique to fentanyl. It is also used for medications like estradiol to treat menopause symptoms, rivastigmine for dementia, and diclofenac for short-term pain from sprains.
Delivery Through the Skin
Once applied, the fentanyl in the patch begins to diffuse through the layers of the skin. This process allows it to enter the systemic circulation, meaning it travels throughout the body via the bloodstream.
Targeting the Central Nervous System
After entering the circulation, fentanyl travels to the brain and spinal cord. There, it selectively binds to specialized sites called mu-opioid receptors.
This action mimics the effects of the body's natural pain-relieving chemicals (endogenous opiates), effectively reducing the sensation of pain.
Why Use a Patch for This Medication?
Consistent and Stable Dosing
The primary advantage of the patch is its ability to provide smooth, predictable drug delivery. It avoids the peaks and troughs in medication levels that can occur with pills taken every few hours.
This stability is critical for managing severe, chronic pain, helping to prevent breakthrough pain and maintain a consistent quality of life.
Bypassing the Digestive System
By absorbing the medication through the skin, the patch avoids the gastrointestinal tract. This can reduce digestive side effects sometimes associated with oral opioid medications.
Convenience and Patient Experience
For long-term pain management, a patch that is changed only every few days is significantly more convenient than a regimen of frequent pills. This improves the overall patient experience and can increase adherence to the treatment plan.
Understanding the Critical Limitations
For Opioid-Tolerant Patients Only
The fentanyl patch is a highly potent medication. It is prescribed exclusively for patients who are already accustomed to taking narcotic pain medications.
Using this patch on someone who is not opioid-tolerant can cause life-threatening respiratory depression. This is its most critical safety limitation.
For Severe and Persistent Pain Only
This tool is designed for managing severe, chronic conditions, such as advanced cancer pain. It is entirely inappropriate for short-term pain from injuries, sprains, or surgery.
The Potency of Fentanyl
Fentanyl is a powerful synthetic opioid. Its use is strictly controlled and monitored by healthcare professionals due to the significant risks involved, which is why it is reserved for specific and severe pain scenarios.
Making the Right Choice for the Goal
The fentanyl patch is a specialized tool with a very narrow and specific purpose. Its application is dictated by the type of pain and the patient's medical history.
- If the primary focus is managing severe, chronic pain in an opioid-tolerant patient: The transdermal patch offers a stable, long-term solution when other options are insufficient.
- If the primary focus is improving convenience and avoiding digestive side effects in long-term therapy: The patch provides a clear advantage over oral medications for suitable candidates.
- If the primary focus is treating acute, minor, or post-surgical pain: The fentanyl patch is dangerously inappropriate and should never be considered for these situations.
Understanding its specific role ensures this powerful tool is used safely and effectively for the patients who need it most.
Summary Table:
| Key Aspect | Description |
|---|---|
| Primary Purpose | Management of severe, persistent, chronic pain in opioid-tolerant patients. |
| Delivery Method | Transdermal (through the skin) for steady, continuous medication release. |
| Key Benefit | Provides consistent, stable dosing, avoiding peaks and troughs of oral medication. |
| Critical Limitation | For use ONLY in patients already tolerant to other opioid pain medications. |
| Ideal For | Long-term conditions like advanced cancer pain, not acute or post-surgical pain. |
Need a reliable transdermal patch solution for your pain management portfolio?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we partner with healthcare and pharmaceutical distributors and brands. Our technical expertise ensures high-quality, consistent delivery systems for potent medications.
Let us help you develop your next product:
- Custom R&D: Tailor patches to specific drug delivery profiles and patient needs.
- Bulk Manufacturing: Scale production with consistent, high-quality output.
Contact our experts today to discuss how we can support your transdermal patch development and manufacturing goals.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Heat Pain Relief Patches Transdermal Patches
People Also Ask
- How should pain relief patches be applied and used? A Guide to Safe & Effective Targeted Relief
- How do pain relief patches provide targeted relief? Discover the Science Behind Effective Pain Management
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief
- How do pain relief patches compare to other pain relief methods? Discover Targeted, Long-Lasting Relief
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief














