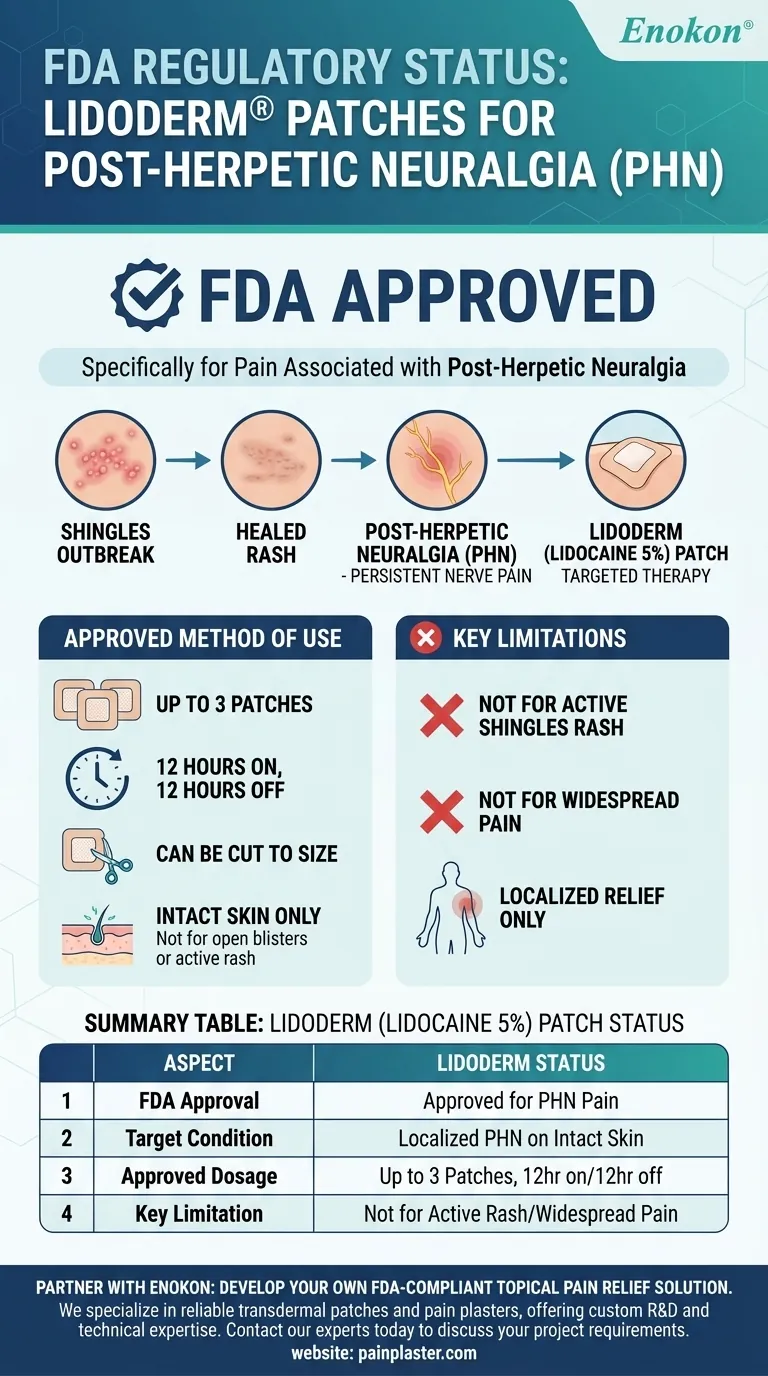
On the regulatory status of Lidoderm, the answer is clear and definitive. The Lidoderm (lidocaine 5%) patch is officially approved by the U.S. Food and Drug Administration (FDA) specifically for the treatment of pain associated with post-herpetic neuralgia (PHN), which is the persistent nerve pain that can linger after a shingles outbreak resolves.
Lidoderm's FDA approval establishes it as a recognized, first-line topical treatment for post-herpetic neuralgia. This specific indication means its primary regulatory standing is as a targeted therapy for localized nerve pain on intact skin, not as a general-purpose pain reliever.
Understanding Lidoderm's Specific FDA Approval
The FDA's approval is not just a simple green light; it defines exactly what a medication is for and how it should be used. For Lidoderm, this approval is highly specific.
The Target Condition: Post-Herpetic Neuralgia (PHN)
Lidoderm is indicated for the relief of pain associated with post-herpetic neuralgia.
This is the chronic, often debilitating nerve pain that can persist for months or even years after the initial shingles rash has healed.
The Mechanism: A Localized Treatment
The patch works by delivering lidocaine, a local anesthetic, directly through the skin to the underlying painful nerves.
This provides targeted relief without the widespread systemic effects that oral pain medications can cause.
The Approved Method of Use
The regulatory approval also outlines the correct and safe way to use the product. Following these guidelines is critical for both efficacy and safety.
Dosage and Duration
The approved regimen is to apply up to three patches to the most painful area of intact skin.
Patches are worn for up to 12 hours within a 24-hour period, followed by a 12-hour rest period without any patches.
Flexibility in Application
The FDA-approved instructions note that patches may be cut into smaller sizes before the protective liner is removed.
This allows you to tailor the patch to fit the specific size and shape of the painful area, ensuring targeted treatment.
Important Patient Considerations
The guidelines recommend using smaller treatment areas for patients who are debilitated or have impaired drug elimination.
This precaution ensures the dose of lidocaine absorbed remains at a safe level for vulnerable individuals.
Understanding the Key Limitations
As a trusted advisor, it's crucial to understand what a product is not approved for. The specificity of Lidoderm's indication highlights its limitations.
Not for an Active Shingles Rash
Lidoderm patches must only be applied to intact skin.
They are not approved for or intended to be used on open blisters or the active rash associated with a shingles outbreak. The indication is for the post-herpetic pain that follows.
A Local vs. Systemic Solution
The patch provides relief only to the area it covers.
It is not a solution for widespread or systemic pain, as its mechanism is entirely localized to the skin and nerves directly beneath the patch.
Making the Right Choice for Your Goal
Understanding the precise regulatory status helps you and your healthcare provider determine if Lidoderm is the appropriate tool for your specific situation.
- If your primary focus is targeted relief for a specific area of post-shingles nerve pain: Lidoderm is an FDA-approved, first-line therapy designed for this exact purpose.
- If your primary focus is managing pain from an active shingles outbreak or widespread body pain: Lidoderm is not the appropriate solution, as its approval is strictly for localized PHN on healed, intact skin.
Ultimately, leveraging an FDA-approved treatment according to its specific indication is the safest and most effective path to managing your condition.
Summary Table:
| Aspect | Lidoderm (Lidocaine 5%) Patch Status |
|---|---|
| FDA Approval | Approved for pain associated with Post-Herpetic Neuralgia (PHN) |
| Target Condition | Localized nerve pain after a shingles rash has healed (intact skin only) |
| Approved Dosage | Up to 3 patches, for up to 12 hours within a 24-hour period |
| Key Limitation | Not approved for use on active shingles blisters or for widespread pain |
Ready to Develop Your Own FDA-Compliant Topical Pain Relief Solution?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we partner with healthcare and pharmaceutical distributors and brands to bring effective treatments to market. Our technical expertise in custom R&D and development ensures your product meets regulatory standards and delivers targeted relief to patients.
Let us help you create a safe and effective topical analgesic patch. Contact our experts today to discuss your project requirements.
Visual Guide
Related Products
- Herbal Eye Protection Patch Eye Patch
- Capsaicin Chili Medicated Pain Relief Patches
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Hydra Gel Health Care Eye Patch
People Also Ask
- What factors should be considered when purchasing eye patches? Essential Guide for Safe & Effective Use
- What are the steps for properly using eye patches? Maximize Benefits for Your Delicate Eye Area
- When should a doctor be consulted regarding the use of this patch? Key Safety Guidelines
- Should under eye patches be applied before or after moisturizer? Optimize Your Skincare Routine
- What are the main benefits of using eye patches in a skincare routine? Revitalize Your Under-Eye Area
















