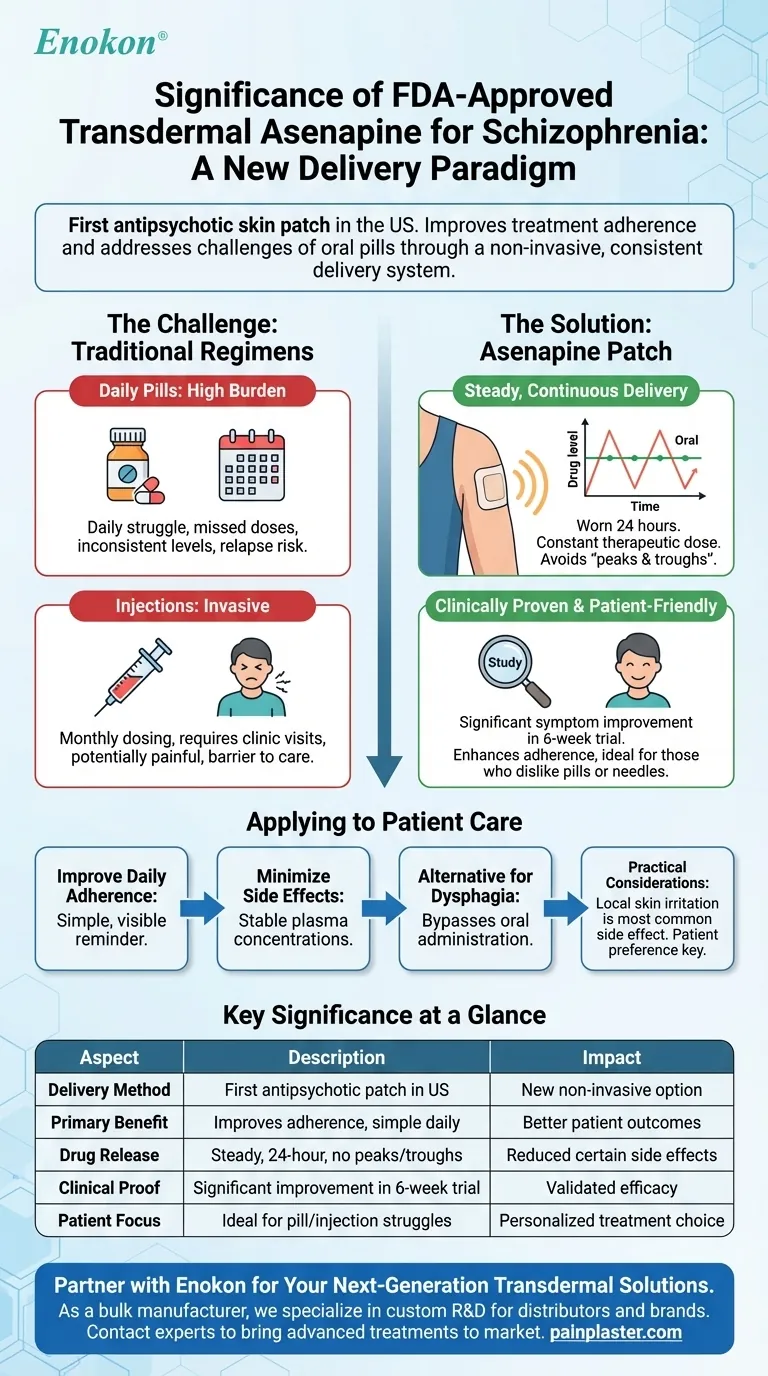
In short, the FDA approval of transdermal asenapine is significant because it is the first antipsychotic medication for schizophrenia available as a skin patch in the United States. This introduces a new, non-invasive method for delivering a proven medication, directly addressing long-standing challenges with treatment adherence and side effects associated with oral pills.
The core significance lies not in a new drug, but in a new delivery system. The transdermal patch offers a way to ensure consistent, steady medication levels, potentially improving patient outcomes for those who struggle with the daily regimen of oral medication.

The Challenge with Traditional Treatment Regimens
For decades, managing schizophrenia has relied primarily on oral medications or long-acting injections. While effective, each method presents distinct challenges for both patients and clinicians.
The Burden of Daily Pills
Taking medication every day can be difficult for anyone, but it is a significant hurdle for individuals managing the cognitive and motivational symptoms of schizophrenia. Missed doses are common and can lead to inconsistent drug levels in the body, which may reduce efficacy and increase the risk of relapse.
The Invasiveness of Injections
Long-acting injectable antipsychotics solve the problem of daily adherence by requiring only monthly or less frequent dosing. However, they require a clinical visit for a potentially painful injection, which can be a significant barrier for some patients.
How the Asenapine Patch Changes the Landscape
The transdermal patch offers a middle ground, combining a simple daily routine with the benefits of continuous drug delivery, bypassing some of the issues seen with oral administration.
A New Delivery System
Transdermal asenapine, worn on the skin, delivers the second-generation antipsychotic asenapine steadily over a 24-hour period. This provides a constant therapeutic dose without the need to swallow a pill.
Consistent Drug Levels
By releasing the drug directly through the skin into the bloodstream, the patch avoids the "peaks and troughs" common with oral medication. This steady release may help reduce certain side effects that are often linked to spikes in drug concentration.
Clinically Proven Efficacy
The FDA's approval was based on a 6-week clinical trial involving over 600 adults with schizophrenia. The transdermal patch demonstrated a statistically significant improvement in symptoms compared to a placebo, as measured by standard clinical scales (PANSS and CGI-S).
Potential for Improved Symptom Control
Research indicates that the steady drug release from a patch can be effective in reducing the negative symptoms of schizophrenia, such as apathy and social withdrawal. It improves mood, thinking, and behavior for those who may not respond optimally to other formulations.
Understanding the Practical Considerations
While this approval marks a significant step forward, it's essential to view it as a new tool in the treatment toolkit, not a universal solution. The choice of medication and delivery system remains highly individual.
A Focus on Patient Preference
The primary advantage of the asenapine patch is its potential to improve treatment adherence. For patients who have difficulty swallowing pills, forget daily doses, or are averse to needles, this provides a valuable and less intrusive alternative.
Potential for Side Effects
Like all transdermal systems, the most common side effect is localized skin irritation at the application site. The systemic side effects are consistent with those of asenapine in its other formulations.
How to Apply This to Patient Care
The availability of transdermal asenapine allows for a more personalized approach to schizophrenia treatment, tailored to the patient's specific needs and challenges.
- If your primary focus is improving daily adherence without injections: The once-daily patch provides a simple, visible reminder that simplifies the treatment regimen.
- If your primary focus is minimizing side effects from fluctuating drug levels: The patch's steady, continuous delivery can help stabilize plasma concentrations.
- If your primary focus is finding an alternative for a patient with dysphagia (difficulty swallowing): The transdermal system completely bypasses the need for oral administration.
This approval expands the options for care, empowering clinicians and patients with a new, effective way to manage schizophrenia.
Summary Table:
| Key Aspect | Significance of the Asenapine Patch |
|---|---|
| Delivery Method | First antipsychotic transdermal patch in the US |
| Primary Benefit | Improves treatment adherence via simple, daily application |
| Drug Release | Provides steady, 24-hour medication levels, avoiding peaks and troughs |
| Clinical Proof | Demonstrated significant symptom improvement in a 6-week trial |
| Patient Focus | Ideal for those who struggle with pills or dislike injections |
Partner with Enokon for Your Next-Generation Transdermal Solutions
The FDA's approval of the asenapine patch highlights the growing demand for innovative, patient-friendly drug delivery systems. As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon is your ideal partner to bring these advanced treatments to market.
We specialize in custom R&D and development for healthcare and pharmaceutical distributors and brands. Benefit from our technical expertise to create effective, high-quality transdermal products that meet specific patient needs.
Ready to develop your own innovative transdermal therapy? Contact our experts today to discuss your project requirements.
Visual Guide
Related Products
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Herbal Eye Protection Patch Eye Patch
People Also Ask
- What makes the cough relief patch a convenient option for managing coughs? A Mess-Free, On-the-Go Solution
- Can pregnant women use pain relief patches? Your Essential Guide to Safe Pain Management
- How does capsaicin work in the Reliever Patch? A Drug-Free Solution for Targeted Pain Relief
- What types of coughs can the far infrared cough relief patch address? Soothe Dry, Wet, and Persistent Coughs
- Are pain relief patches safe for sensitive skin? Your Guide to Safe Use & Skin Testing















