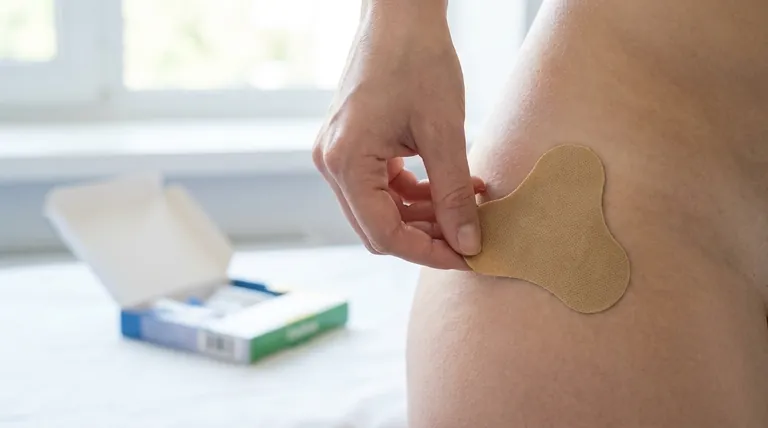
Transdermal methylphenidate is a medication prescribed primarily to treat attention deficit hyperactivity disorder (ADHD). Delivered via a skin patch, this stimulant medication is designed to improve attention while reducing the common ADHD symptoms of impulsivity and hyperactivity. It may also be prescribed for other conditions as determined by a healthcare professional.
The transdermal patch offers a method for delivering a steady, controlled dose of methylphenidate through the skin over several hours. However, its effectiveness and safety are entirely dependent on strict adherence to the correct application and handling protocols.
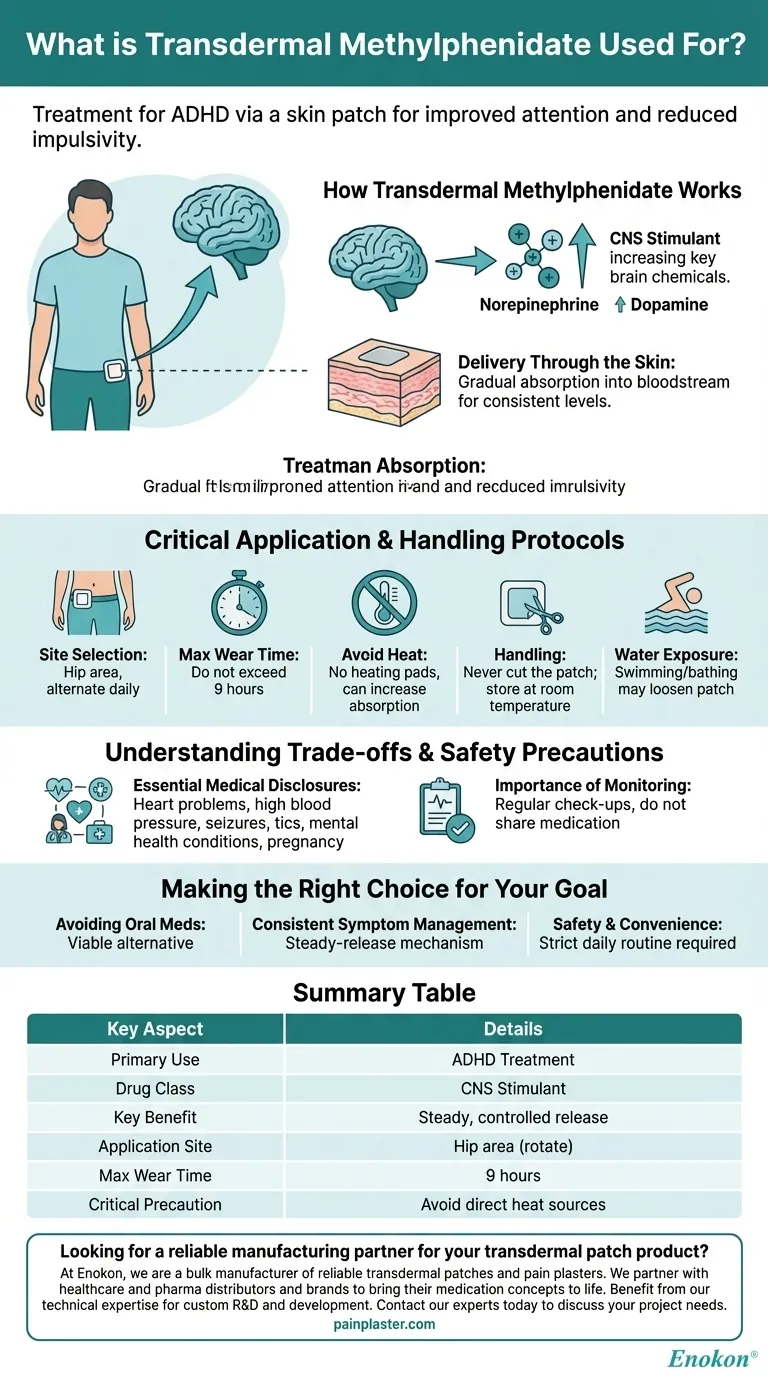
How Transdermal Methylphenidate Works
A Stimulant for Brain Chemistry
Transdermal methylphenidate belongs to a class of drugs known as central nervous system (CNS) stimulants.
It is believed to work by increasing the levels of key brain chemicals, specifically norepinephrine and dopamine. These neurotransmitters play a crucial role in the parts of the brain responsible for attention, focus, and impulse control.
Delivery Through the Skin
The "transdermal" aspect refers to the delivery method—a patch applied directly to the skin.
This system allows the medication to be absorbed gradually into the bloodstream over a set period, providing a consistent level of the drug without the peaks and troughs that can sometimes occur with oral pills.
Critical Application and Handling Protocols
Proper use is essential for the medication to work safely and effectively. The patch must be applied precisely as directed by a healthcare provider.
Site Selection and Duration
The patch should be applied to the hip area.
To prevent skin irritation, it is crucial to alternate application sites daily, never placing a new patch on the exact same spot as the day before. The patch should not be worn for more than 9 hours.
Environmental and Physical Factors
You must avoid exposing the patch to direct heat sources, such as heating pads or electric blankets, as this can increase the rate of drug absorption to dangerous levels.
Activities like swimming or bathing may loosen the patch, reducing its effectiveness. If the patch does become loose, do not attempt to re-secure it with tape or other adhesives.
Handling and Storage
You should never cut the patch, as this can damage the medication delivery system and result in an incorrect dose.
Patches should be stored at room temperature in their sealed pouches until they are ready to be used. Keep them safely out of reach of children and pets.
Understanding the Trade-offs and Safety Precautions
While effective, this medication requires a high degree of caution and full disclosure with your medical team.
Essential Medical Disclosures
Before starting treatment, it is critical to inform your doctor about any personal or family history of specific conditions.
These include heart problems, high blood pressure, seizures, circulation issues, tics or Tourette's syndrome, and mental health conditions like depression or bipolar disorder. You must also disclose if you are pregnant, planning to become pregnant, or breastfeeding.
The Importance of Monitoring
This medication should never be shared with anyone else.
Regular medical appointments are necessary to monitor its effects, check for side effects, and ensure the treatment plan remains appropriate for your needs.
Making the Right Choice for Your Goal
- If your primary focus is avoiding oral medication: The transdermal patch offers a viable alternative for individuals who have difficulty swallowing pills.
- If your primary focus is consistent symptom management: The steady-release mechanism can help maintain stable medication levels throughout its application time.
- If your primary focus is safety and convenience: You must be prepared to follow a strict daily routine of application, removal, and site rotation to prevent skin issues and ensure proper dosing.
Understanding how to use this medication correctly is just as important as knowing what it's for, ensuring both safety and effectiveness in managing ADHD.
Summary Table:
| Key Aspect | Details |
|---|---|
| Primary Use | Treatment of Attention Deficit Hyperactivity Disorder (ADHD) |
| Drug Class | Central Nervous System (CNS) Stimulant |
| Key Benefit | Steady, controlled drug release over several hours |
| Application Site | Hip area (must be rotated daily) |
| Max Wear Time | 9 hours |
| Critical Precaution | Avoid direct heat sources (e.g., heating pads) |
Looking for a reliable manufacturing partner for your transdermal patch product?
At Enokon, we are a bulk manufacturer of reliable transdermal patches and pain plasters. We partner with healthcare and pharma distributors and brands to bring their medication concepts to life.
Benefit from our technical expertise for custom R&D and development, ensuring a product that is safe, effective, and meets your exact specifications.
Contact our experts today to discuss your project needs.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Icy Hot Menthol Medicine Pain Relief Patch
- Menthol Gel Pain Relief Patch
People Also Ask
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- What are pain relief patches and how are they used? A Guide to Safe, Targeted Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief















