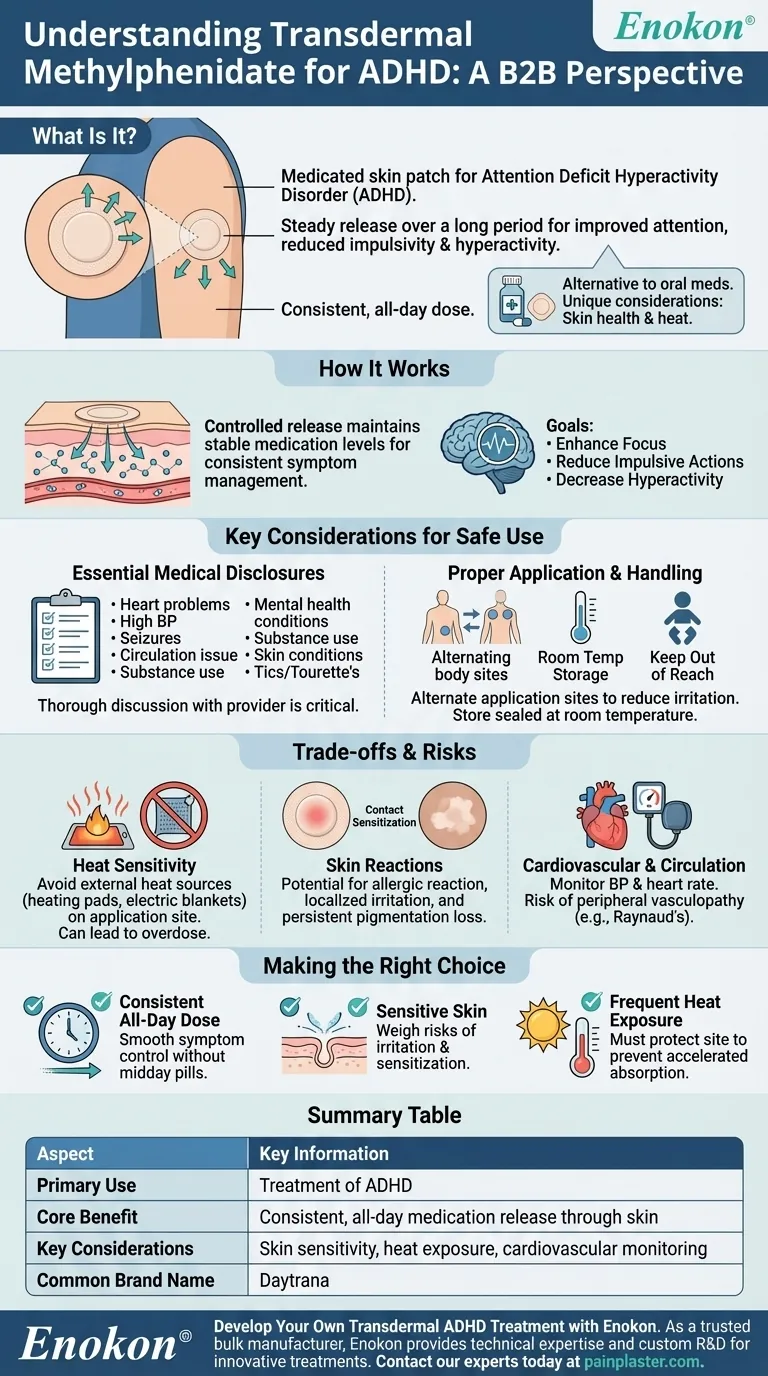
At its core, transdermal methylphenidate is a medicated skin patch used to treat Attention Deficit Hyperactivity Disorder (ADHD). This delivery system is designed to release the medication steadily through the skin over a long period, helping to improve attention and reduce the impulsivity and hyperactivity characteristic of ADHD.
While oral medications are common for ADHD, the transdermal patch offers an alternative way to deliver methylphenidate. Its primary advantage is providing a consistent, all-day dose, but this method introduces unique considerations, particularly regarding skin health and heat exposure.

How Transdermal Delivery Works for ADHD
The Principle of a Medicated Patch
Transdermal methylphenidate, known by brand names like Daytrana, uses a patch that adheres to the skin. The patch contains the medication, which is slowly absorbed through the skin's layers and into the bloodstream.
This method bypasses the digestive system and provides a controlled release of the drug throughout the day. The goal is to maintain a stable level of medication in the body for consistent symptom management.
The Goal: Managing Core ADHD Symptoms
The ultimate purpose of the medication is to manage the core symptoms of ADHD. By helping to regulate brain activity, it aims to enhance focus, reduce impulsive actions, and decrease hyperactivity in both children and adults.
Key Considerations for Safe and Effective Use
Essential Medical Disclosures
Before starting treatment, it is critical to have a thorough discussion with your healthcare provider. You must disclose any personal or family history of heart problems, high blood pressure, seizures, or circulation issues like Raynaud's phenomenon.
It is also vital to mention any mental health conditions, substance use history, skin conditions, tics, or Tourette's syndrome. This information allows your doctor to assess if the medication is appropriate and safe for you.
Proper Application and Handling
The patch must be applied correctly to function as intended. Always alternate application sites on the body to reduce the risk of skin irritation.
Keep patches in their sealed pouches until you are ready to use them. They should be stored at room temperature, never refrigerated or frozen, and kept safely out of reach of children.
Understanding the Trade-offs and Risks
The Impact of External Heat
A unique risk associated with a transdermal patch is its sensitivity to heat. You must avoid exposing the application site to direct external heat sources, such as heating pads or electric blankets.
Heat can increase the rate at which the medication is absorbed into the bloodstream, potentially leading to an overdose.
Potential for Skin Reactions
Because the medication is delivered through the skin, there is a risk of localized skin reactions. This can include contact sensitization, an allergic reaction to the patch components.
In some cases, users may experience a persistent loss of skin pigmentation (color) at the application sites.
Cardiovascular and Circulatory Monitoring
Like oral stimulants, transdermal methylphenidate can increase blood pressure and heart rate. Your doctor will need to monitor these vital signs regularly.
There is also a risk of peripheral vasculopathy, including Raynaud's phenomenon, where blood flow to fingers and toes is restricted.
Other Systemic Side Effects
While the delivery method is different, the medication still circulates throughout the body. Other potential side effects that require monitoring include slowed growth in children, blurred vision, and a lowered seizure threshold in patients with a history of seizures.
Making the Right Choice for Your Treatment Plan
A transdermal patch is one of several tools available for managing ADHD. Understanding its specific profile is key to a successful treatment strategy.
- If your primary focus is a smooth, all-day dose without remembering midday pills: The transdermal patch can be an excellent option for providing consistent symptom control.
- If you or your child have sensitive skin or existing skin conditions: You must carefully weigh the risk of skin irritation, sensitization, and pigmentation loss with your doctor.
- If your lifestyle involves frequent exposure to high heat or direct sun: Be aware that the patch site must be protected to prevent accelerated medication absorption.
Ultimately, the decision to use transdermal methylphenidate should be made in close partnership with your healthcare provider to ensure it aligns with your specific needs and health profile.
Summary Table:
| Aspect | Key Information |
|---|---|
| Primary Use | Treatment of Attention Deficit Hyperactivity Disorder (ADHD) |
| Core Benefit | Provides consistent, all-day medication release through the skin |
| Key Considerations | Skin sensitivity, heat exposure, cardiovascular monitoring |
| Common Brand Name | Daytrana |
Develop Your Own Transdermal ADHD Treatment with Enokon
As a trusted bulk manufacturer of reliable transdermal patches, Enokon provides the technical expertise and custom R&D capabilities to help healthcare and pharmaceutical brands bring innovative treatments to market. If you are developing a medicated patch for conditions like ADHD, our team can support your project from formulation to final product.
Contact our experts today to discuss how we can partner on your next transdermal patch development.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Capsaicin Chili Medicated Pain Relief Patches
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- How do pain relief patches compare to other pain relief methods? Discover Targeted, Long-Lasting Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief
- How effective are pain relief patches for muscle pain? Target Localized Pain with Transdermal Delivery
- How do pain relief patches provide targeted relief? Discover the Science Behind Effective Pain Management














