Before using the methylphenidate transdermal system, it is critical to discuss your complete medical history with your doctor. A range of pre-existing conditions, particularly those affecting the heart, circulation, and mental health, can be significantly worsened by this medication. Other concerns include seizure disorders, skin conditions like vitiligo, and a personal or family history of tics.
The core issue is that methylphenidate is a stimulant. It can place stress on the cardiovascular system and alter brain chemistry, potentially exacerbating underlying conditions. The transdermal patch introduces additional risks related to skin reactions and heat exposure.
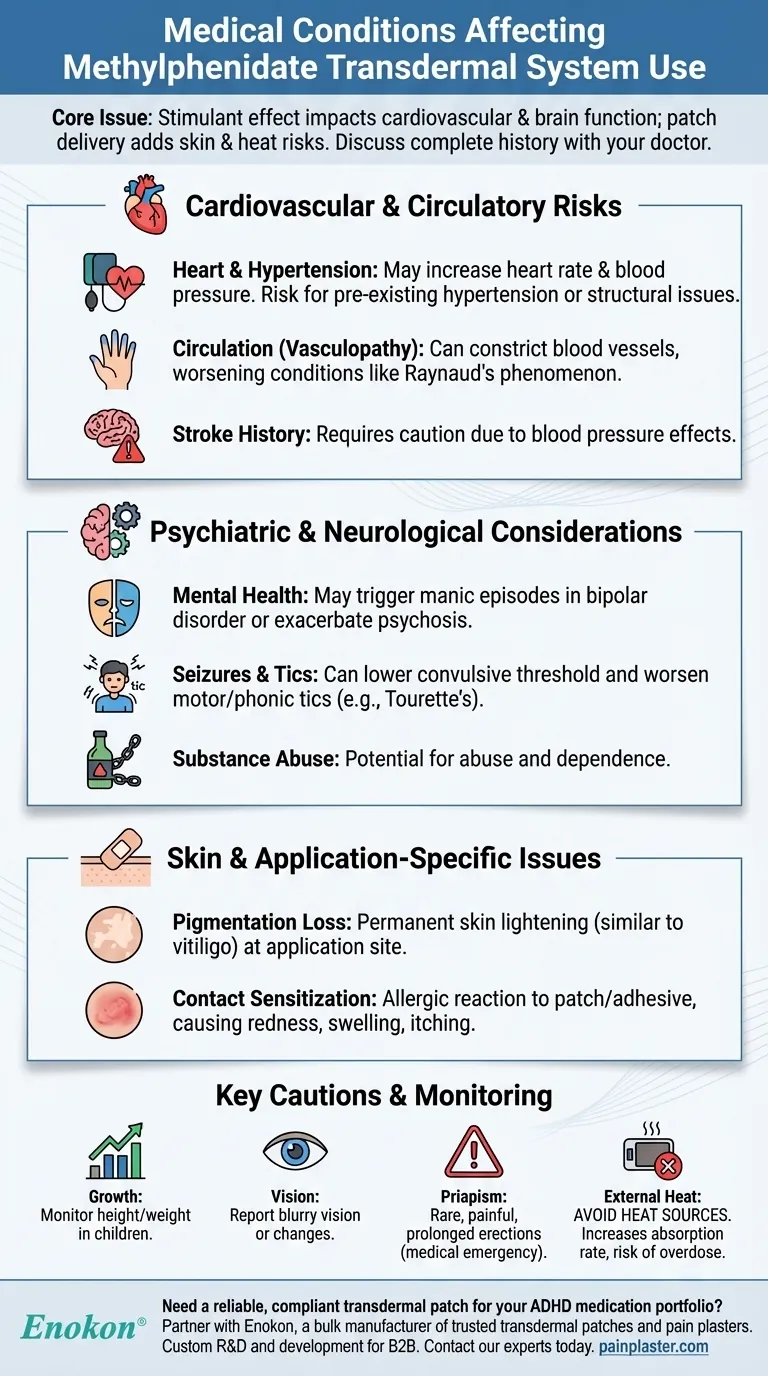
Cardiovascular and Circulatory Risks
As a stimulant, methylphenidate can directly impact your heart and blood vessels. This makes a thorough cardiovascular assessment essential before starting treatment.
Heart Conditions and Hypertension
Methylphenidate is known to cause modest increases in average blood pressure and heart rate. For individuals with pre-existing hypertension or structural heart abnormalities, these changes can pose a significant risk.
Circulation Problems (Vasculopathy)
Patients with peripheral vasculopathy, including Raynaud's phenomenon, may experience worsened symptoms. The medication can constrict blood vessels, reducing blood flow to the fingers and toes.
History of Stroke
Given its effect on blood pressure, the medication requires caution in patients with a history of stroke or other cerebrovascular problems.
Psychiatric and Neurological Considerations
The central nervous system effects of methylphenidate can interact with or worsen several neurological and psychiatric disorders.
Pre-existing Mental Health Conditions
For individuals with bipolar disorder, stimulants can trigger manic episodes. In patients with a history of psychosis, the medication may exacerbate symptoms like hallucinations or delusions.
Seizure Disorders
Methylphenidate can lower the convulsive threshold. This means individuals with a history of seizures may be at a higher risk of having one while on the medication.
Tics and Tourette Syndrome
The medication can cause or worsen motor and phonic tics. Patients with Tourette syndrome or a family history of the condition require careful monitoring.
History of Substance Abuse
Due to its nature as a stimulant, methylphenidate has a potential for abuse and dependence. A history of alcohol or drug abuse must be carefully considered.
Skin and Application-Specific Issues
The transdermal patch delivery system introduces unique risks that are not present with oral formulations.
Loss of Skin Pigmentation
Persistent loss of skin pigmentation, a condition similar to vitiligo, has been reported at the sites where the patch is applied. This skin lightening can be permanent.
Contact Sensitization
Some individuals may develop an allergic skin reaction (contact sensitization) to the medication or the patch adhesive. This can manifest as intense redness, swelling, and itching.
Understanding the Key Cautions
Beyond specific contraindications, several factors require ongoing monitoring and awareness during treatment.
Monitoring Growth in Children
In pediatric patients, the use of stimulants must be monitored for its potential impact on growth. Height and weight should be tracked regularly.
Vision Changes
Some patients report difficulties with visual accommodation and blurry vision. Any significant changes to eyesight should be reported to a doctor.
Risk of Priapism
Though rare, methylphenidate has been associated with prolonged and painful erections (priapism), which is a medical emergency that requires immediate attention.
Avoiding External Heat
Applying an external heat source, such as a heating pad or electric blanket, to the patch area is dangerous. Heat dramatically increases the rate of drug absorption, which can lead to an overdose.
Making the Right Choice with Your Doctor
A transparent discussion of your entire health history is the only way to ensure this medication is both safe and effective for you.
- If your primary focus is managing ADHD with a history of heart or circulation problems: Careful and continuous monitoring of your blood pressure and heart rate is non-negotiable.
- If you have a history of mental health conditions like bipolar disorder or psychosis: It is crucial to monitor for any changes in mood, behavior, or the emergence of psychotic symptoms.
- If you have a history of seizures or tics: You and your doctor must weigh the risk of worsening these conditions against the potential benefits of the medication.
- If you have sensitive skin or a history of skin conditions: You must diligently alternate patch sites and watch closely for any signs of severe skin reaction or pigmentation loss.
Disclosing every aspect of your health history empowers your physician to make the safest and most effective treatment decision for your specific needs.
Summary Table:
| Condition Category | Specific Conditions to Disclose | Potential Risk |
|---|---|---|
| Cardiovascular | Hypertension, Heart Disease, Stroke, Raynaud's | Increased blood pressure, heart rate, reduced blood flow |
| Psychiatric/Neurological | Bipolar Disorder, Psychosis, Seizures, Tics/Tourette's | Manic episodes, worsened symptoms, lower seizure threshold |
| Skin-Related | Vitiligo, Skin Allergies, Sensitive Skin | Permanent skin lightening, contact dermatitis, irritation |
| Other Key Factors | Substance Abuse History, Growth in Children, Priapism | Risk of dependence, monitor height/weight, medical emergency |
Need a reliable, compliant transdermal patch for your ADHD medication portfolio? Partner with Enokon, a bulk manufacturer of trusted transdermal patches and pain plasters. We specialize in custom R&D and development for healthcare and pharmaceutical distributors and brands. Our technical expertise ensures your product meets the highest safety and efficacy standards for sensitive patient populations. Contact our experts today to discuss your custom patch development needs.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Medical Cooling Gel Patches for Fever Cooling Patches
People Also Ask
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- How should pain relief patches be applied and used? A Guide to Safe & Effective Targeted Relief
- How effective are pain relief patches for muscle pain? Target Localized Pain with Transdermal Delivery
- How do pain relief patches compare to other pain relief methods? Discover Targeted, Long-Lasting Relief













