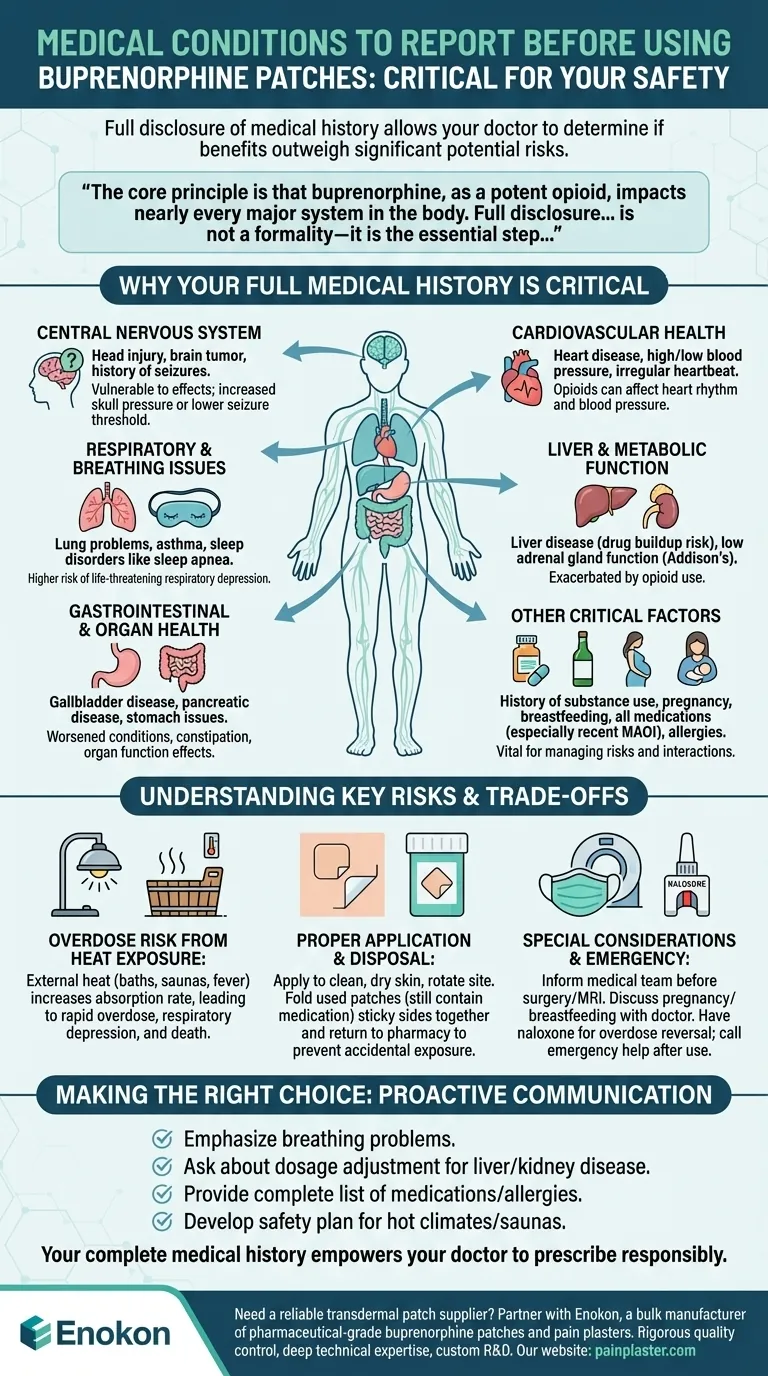
Before using buprenorphine patches, you must provide your healthcare provider with a complete and honest medical history. It is critical to report any history of lung or breathing problems, head injuries, brain tumors, heart conditions, liver or gallbladder disease, stomach issues, seizures, substance or alcohol use, and mental health conditions. Also disclose all allergies, medications (especially recent MAOI use), and if you are pregnant or breastfeeding.
The core principle is that buprenorphine, as a potent opioid, impacts nearly every major system in the body. Full disclosure of your medical history is not a formality—it is the essential step that allows your doctor to determine if the benefits of this medication outweigh its significant potential risks for you.

Why Your Full Medical History is Critical
Buprenorphine works by acting on the central nervous system to block pain signals. Because this system regulates everything from breathing to heart rate, any pre-existing condition can dramatically alter how your body responds to the medication, increasing the risk of severe complications.
Central Nervous System Conditions
Conditions like a head injury, brain tumor, or history of seizures make you more vulnerable to buprenorphine's effects on the brain. The medication can increase pressure inside the skull or lower the seizure threshold, creating a dangerous situation.
Respiratory and Breathing Issues
This is one of the most critical areas of concern. Buprenorphine can suppress the drive to breathe, a condition known as respiratory depression.
If you have underlying lung problems, asthma, or sleep disorders like sleep apnea, your risk of life-threatening breathing complications is significantly higher.
Cardiovascular Health
You must report any history of heart disease, high or low blood pressure, or an irregular heartbeat. Opioids can affect heart rhythm and blood pressure, and for individuals with existing cardiac issues, these changes can be dangerous.
Liver and Metabolic Function
The liver is responsible for processing buprenorphine. If you have liver disease, the drug can build up in your system to toxic levels, increasing the risk of overdose and other side effects.
Similarly, conditions like low adrenal gland function (Addison's disease) can be exacerbated by opioid use.
Gastrointestinal and Organ Health
Inform your doctor about any gallbladder disease, pancreatic disease, or other stomach and intestinal problems. Opioids can worsen these conditions, particularly by causing constipation or affecting organ function.
History of Substance Use
A history of drug or alcohol addiction is vital information for your doctor. This context helps them manage risks related to dependence, misuse, and potential interactions.
Understanding the Key Risks and Trade-offs
Using a buprenorphine patch requires careful management to avoid serious harm. Understanding the primary risks allows you to be an active partner in your own safety.
The Overdose Risk from Heat Exposure
External heat sources are extremely dangerous when using the patch. Hot baths, saunas, heating pads, or even a high fever can increase the rate at which the drug is absorbed into your bloodstream.
This rapid absorption can easily lead to an accidental overdose, causing severe respiratory depression, unresponsiveness, and death.
Proper Application and Disposal
The patch must be applied to clean, dry, non-irritated skin, and the application site should be rotated every time. A new patch should not be applied to the same spot for at least 3-4 weeks.
Used patches still contain a significant amount of medication. They must be folded in half with the sticky sides together and returned to a pharmacy for safe disposal to prevent accidental exposure to others, especially children.
Special Considerations: Surgery and Pregnancy
You must inform any medical or surgical team that you are using a buprenorphine patch before any procedure, including surgery or an MRI. The patch may need to be removed.
If you are pregnant, planning to become pregnant, or breastfeeding, discuss patch use with your doctor. The medication can affect the fetus or pass into breast milk.
Emergency Preparedness with Naloxone
Given the risk of overdose, your doctor may prescribe naloxone, an emergency medication that can reverse opioid effects. Ensure you and your family know where it is and how to use it, and always call for emergency medical help after its administration.
Making the Right Choice for Your Safety
Proactive communication with your healthcare provider is the single most important factor in using a buprenorphine patch safely.
- If you have any history of breathing problems: Emphasize this to your doctor, as you are at the highest risk for dangerous side effects.
- If you have a history of liver or kidney disease: Ask your doctor if your dosage needs to be adjusted to prevent the medication from building up to toxic levels.
- If you take any other medications (including over-the-counter): Provide a complete list to your pharmacist and doctor to screen for dangerous interactions.
- If you live in a hot climate or use saunas/hot tubs: Discuss the significant overdose risk with your doctor and develop a clear safety plan.
Ultimately, your complete medical history empowers your doctor to prescribe this medication responsibly and keep you safe.
Summary Table:
| Medical Condition Category | Specific Conditions to Report |
|---|---|
| Central Nervous System | Head injury, brain tumor, seizures |
| Respiratory System | Asthma, COPD, sleep apnea |
| Cardiovascular System | Heart disease, high/low blood pressure, irregular heartbeat |
| Liver & Metabolic | Liver disease, Addison's disease |
| Gastrointestinal | Gallbladder disease, pancreatic disease |
| Other Critical Factors | Substance use history, pregnancy, breastfeeding, all medications/allergies |
Need a reliable transdermal patch supplier? Partner with Enokon, a bulk manufacturer of pharmaceutical-grade buprenorphine patches and pain plasters. We combine rigorous quality control with deep technical expertise to support healthcare distributors and brands with custom R&D and development. Ensure your patients receive safe, effective, and consistent medication delivery. Contact our experts today to discuss your requirements.
Visual Guide
Related Products
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Icy Hot Menthol Medicine Pain Relief Patch
- Menthol Gel Pain Relief Patch
People Also Ask
- How do you apply the Signal Relief patch to find the proper placement? A Step-by-Step Guide to Maximum Relief
- Can the pain relief patch be used with other external analgesic products? A Critical Safety Guide
- What is the purpose of capsaicin patches? A Guide to Temporary Pain Relief
- What side effects might occur from using capsaicin patches? Understand the difference between normal sensation and danger signs.
- What precautions should be taken with buprenorphine patches? Ensure Safe Use and Avoid Overdose Risks















