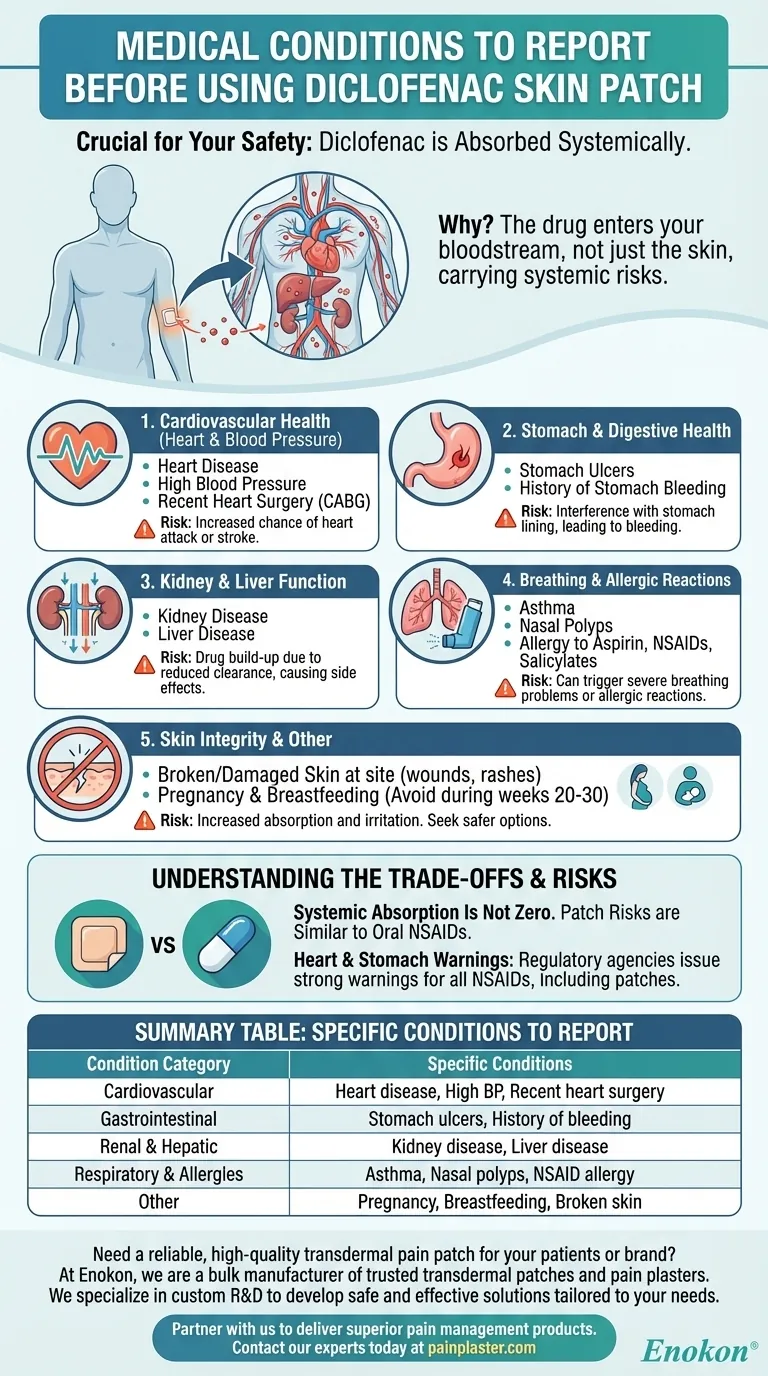
Before using a diclofenac skin patch, you must inform your doctor about a range of medical conditions to ensure it is safe for you. The most critical conditions to report include any history of heart disease, recent heart surgery, high blood pressure, stomach ulcers or bleeding, asthma, kidney or liver disease, and any allergies to NSAIDs like aspirin or ibuprofen. It is also crucial to disclose if you are pregnant, planning a pregnancy, or breastfeeding.
The core reason for this disclosure is that diclofenac, even when applied to the skin, is absorbed into your bloodstream. This means it can cause systemic side effects and worsen pre-existing health problems, particularly those related to your heart, stomach, and kidneys.

Why Your Full Medical History Matters for a Skin Patch
The Drug Enters Your System
Diclofenac is a Nonsteroidal Anti-Inflammatory Drug (NSAID). While a patch delivers the medication locally to relieve pain, a portion of the drug is absorbed through your skin and circulates throughout your body.
This systemic absorption means the patch can carry risks similar to those of oral NSAID pills, making your full medical history essential for safe use.
Key Conditions to Discuss With Your Doctor
Cardiovascular Health (Heart and Blood Pressure)
All NSAIDs, including diclofenac, can increase the risk of serious cardiovascular events like a heart attack or stroke.
You must inform your doctor if you have heart disease, high blood pressure, or have recently had coronary artery bypass graft (CABG) surgery. These conditions put you at a higher risk for complications.
Stomach and Digestive Health
NSAIDs can interfere with the protective lining of your stomach, which can lead to ulcers and internal bleeding.
A prior history of stomach ulcers or bleeding significantly increases the risk of this happening again, even with a topical patch.
Kidney and Liver Function
Your kidneys and liver are responsible for processing and clearing medications from your body.
If you have kidney disease or liver disease, the drug may not be cleared effectively. This can cause it to build up in your system, increasing the risk of side effects.
Breathing and Allergic Reactions
Hypersensitivity to NSAIDs is a serious concern.
Tell your doctor if you have asthma, nasal polyps, or a known allergy to aspirin, other NSAIDs, or salicylates. Using diclofenac could trigger a severe breathing problem or allergic reaction.
Skin Integrity at the Application Site
The patch is designed to be used only on healthy, unbroken skin.
Never apply the patch to skin that has open wounds, infections, rashes, or any other form of damage. Doing so can increase drug absorption and cause significant local irritation.
Understanding the Trade-offs and Risks
Systemic Absorption Is Not Zero
The primary trade-off of a patch is convenience for a risk that is lower, but not eliminated. Do not assume a skin patch is only a local treatment. It delivers medication system-wide and carries the potential for serious side effects.
Heart and Stomach Warnings
Regulatory agencies issue strong warnings for NSAIDs regarding the increased risk of heart attack, stroke, and stomach bleeding. These risks apply to the diclofenac patch.
Proper Application is Critical for Safety
Misusing the patch can increase risks. Always wear only one patch at a time and apply it to clean, dry, intact skin. Remove the old patch before applying a new one, and wash your hands after handling it.
Making the Right Choice for Your Health
To use the diclofenac patch safely, a transparent conversation with your healthcare provider is essential.
- If your primary focus is pain relief with a history of heart issues: You must discuss the cardiovascular risks, as your doctor may need to monitor you closely or recommend an alternative.
- If your primary focus is pain relief with a sensitive stomach: Your provider needs to weigh the benefit against the significant risk of gastrointestinal bleeding.
- If your primary focus is pain relief and you have asthma or NSAID allergies: You are at high risk for a severe reaction and will likely need a different class of medication.
- If you are pregnant or planning to be: You should avoid using the patch, especially between weeks 20 and 30 of pregnancy, and seek safer pain relief options from your doctor.
Ultimately, providing a complete medical history empowers your doctor to determine if the diclofenac patch is the safest and most effective choice for your specific situation.
Summary Table:
| Condition Category | Specific Conditions to Report |
|---|---|
| Cardiovascular | Heart disease, high blood pressure, recent heart surgery |
| Gastrointestinal | Stomach ulcers, history of stomach bleeding |
| Renal & Hepatic | Kidney disease, liver disease |
| Respiratory & Allergies | Asthma, nasal polyps, allergy to aspirin/NSAIDs |
| Other | Pregnancy, breastfeeding, broken/damaged skin at application site |
Need a reliable, high-quality transdermal pain patch for your patients or brand?
At Enokon, we are a bulk manufacturer of trusted transdermal patches and pain plasters. We specialize in custom R&D to develop safe and effective solutions tailored to your needs.
Partner with us to deliver superior pain management products.
Contact our experts today to discuss your requirements and benefit from our technical expertise.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Capsaicin Chili Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Pain Patch Relief Pain Reliever for Back
People Also Ask
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- How effective are pain relief patches for muscle pain? Target Localized Pain with Transdermal Delivery
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief
- How do pain relief patches compare to other pain relief methods? Discover Targeted, Long-Lasting Relief














