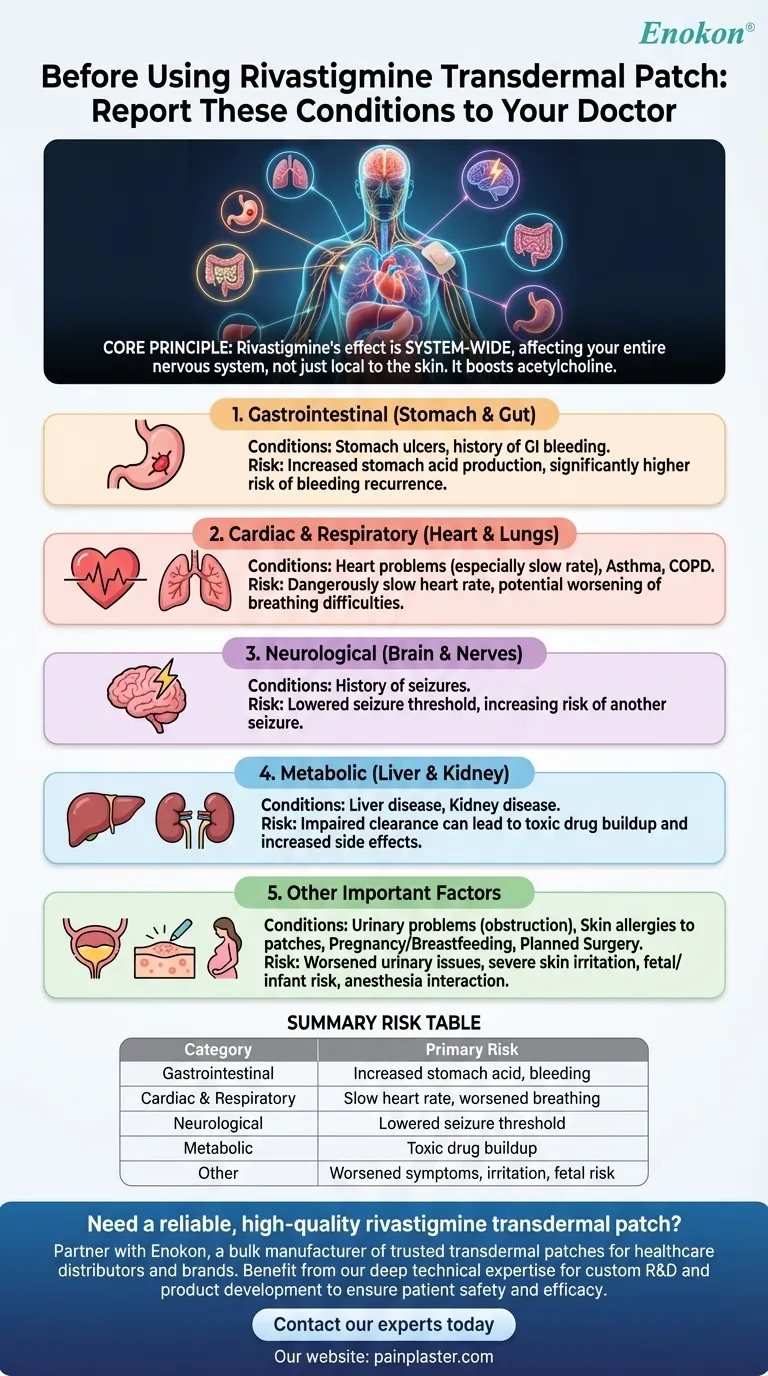
Before using a rivastigmine transdermal patch, you must report a comprehensive range of medical conditions to your doctor to ensure your safety. The most critical conditions include any history of stomach ulcers or bleeding, seizures, heart problems (especially slow heart rate), respiratory disorders like asthma or COPD, liver or kidney disease, and any problems with urination.
The core principle to understand is that rivastigmine is not just a skin treatment; it is absorbed into your bloodstream and affects your entire nervous system. Therefore, any pre-existing condition related to the heart, lungs, stomach, or brain must be carefully evaluated before starting therapy.

Why Your Full Medical History is Crucial
The rivastigmine patch works by increasing the levels of a chemical messenger in the brain, which helps with symptoms of dementia. However, this chemical also plays a role in many other bodily functions, creating potential risks if you have certain underlying health issues.
The Mechanism's System-Wide Impact
Rivastigmine is a cholinesterase inhibitor. It boosts the activity of a neurotransmitter called acetylcholine throughout your body's nervous system.
This system-wide effect is why a condition in one part of your body can be negatively impacted by a medication designed to work on your brain.
Increased Risk of Stomach Ulcers
The same mechanism that helps the brain can also increase the production of stomach acid.
If you have a history of ulcers or gastrointestinal bleeding, this puts you at a significantly higher risk for a recurrence.
Impact on the Heart and Lungs
Acetylcholine helps regulate your heart rate and the constriction of your airways.
For individuals with existing heart problems, rivastigmine can cause a dangerously slow heart rate. In those with asthma or COPD, it can potentially worsen breathing difficulties.
Lowering the Seizure Threshold
Any medication that alters brain chemistry can potentially affect the brain's electrical stability.
If you have a history of seizures, it is critical to report it, as rivastigmine could increase the risk of having another one.
Liver and Kidney Function
Your liver and kidneys are responsible for processing and clearing the medication from your body.
If these organs are impaired due to disease, the drug can build up to toxic levels, increasing the severity of all potential side effects.
Complications with Urination
The chemical messenger affected by rivastigmine also plays a role in bladder control.
For individuals with pre-existing urinary obstruction or prostate issues, the medication could potentially make these problems worse.
Understanding the Broader Risks
Beyond the primary organ systems, other factors must be considered to use the patch safely and effectively.
Skin Allergies and Sensitivities
Your doctor must know about any history of allergic reactions to other medicated skin patches.
An allergic reaction to the patch adhesive or the drug itself can cause severe skin irritation and may mean a transdermal route is not right for you.
Pregnancy, Breastfeeding, and Surgery
It is standard and vital to inform your doctor if you are pregnant, planning to become pregnant, or breastfeeding.
You must also notify any doctor or dentist that you are using rivastigmine before any planned surgery, as it can interact with anesthesia and other medications.
Making the Right Choice for Your Safety
Proactive communication is the key to minimizing risk. Your complete medical history allows your healthcare provider to weigh the benefits of rivastigmine against its potential dangers for your specific situation.
- If your primary concern is a history of stomach ulcers: Your doctor must monitor you closely for signs of gastrointestinal bleeding.
- If you have a known heart or lung condition: Careful monitoring of your heart rate and respiratory status is non-negotiable.
- If you have compromised liver or kidney function: Your doctor may need to start with a lower dose and adjust it much more slowly.
- If you have a history of seizures or urinary problems: Discussing these conditions is critical, as rivastigmine could potentially exacerbate them.
Ultimately, providing a complete and honest medical history is the single most important step you can take to ensure this medication helps you safely.
Summary Table:
| Condition Category | Specific Conditions to Report | Primary Risk |
|---|---|---|
| Gastrointestinal | Stomach ulcers, GI bleeding | Increased stomach acid, bleeding risk |
| Cardiac & Respiratory | Heart problems (slow rate), Asthma, COPD | Dangerously slow heart rate, worsened breathing |
| Neurological | History of seizures | Lowered seizure threshold |
| Metabolic | Liver or kidney disease | Toxic drug buildup due to impaired clearance |
| Other | Urinary problems, skin allergies to patches, pregnancy/breastfeeding | Worsened symptoms, severe skin irritation, fetal/ infant risk |
Need a reliable, high-quality rivastigmine transdermal patch? Partner with Enokon, a bulk manufacturer of trusted transdermal patches and pain plasters for healthcare and pharmaceutical distributors and brands. Benefit from our deep technical expertise for custom R&D and product development to ensure patient safety and efficacy. Contact our experts today to discuss your needs.
Visual Guide
Related Products
- Herbal Eye Protection Patch Eye Patch
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Menthol Gel Pain Relief Patch
People Also Ask
- What are the steps for applying under-eye patches? Boost Your Eye Care Routine
- How quickly can you see results from using under eye patches? Instant Brightening & Long-Term Benefits
- What factors should be considered when purchasing eye patches? Essential Guide for Safe & Effective Use
- What are the steps for properly using eye patches? Maximize Benefits for Your Delicate Eye Area
- How can using eye patches contribute to a self-care skincare routine? Boost Hydration & Relaxation














