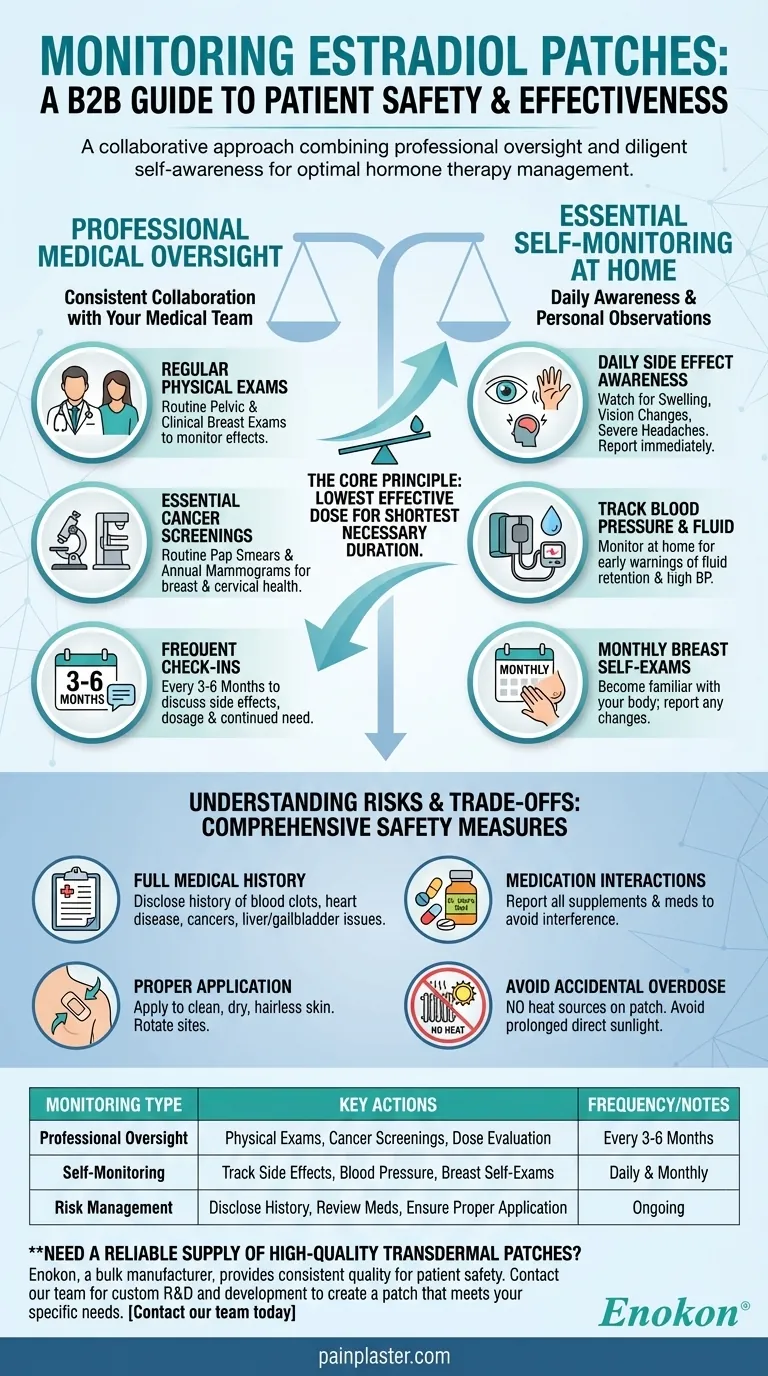
When using estradiol patches, monitoring is a combination of regular professional medical checkups and diligent self-awareness. This includes routine pelvic exams, Pap smears, and mammograms, alongside closely watching for symptoms like fluid retention, high blood pressure, swelling, or changes in vision.
The core principle of monitoring is to ensure you receive the lowest effective dose for the shortest necessary duration, making it a collaborative effort between you and your healthcare provider to maximize benefits while proactively managing risks.
Professional Medical Oversight
Effective hormone therapy is not a "set it and forget it" treatment. It requires consistent partnership with your medical team to track your body's response and adjust as needed.
Regular Physical Examinations
Your doctor will need to perform routine physical exams to monitor the effects of estradiol. This typically includes clinical breast exams and pelvic exams.
Essential Cancer Screenings
Regular screenings are a critical safety measure. This includes routine Pap smears as recommended by your doctor and annual mammograms to monitor breast health.
Frequent Medical Check-Ins
You should have regular checkups with your healthcare provider, often every 3 to 6 months. These appointments are essential for discussing any side effects, evaluating the effectiveness of your current dose, and determining if continued treatment is necessary.
Essential Self-Monitoring at Home
Your personal observations are one of the most important sources of information. Paying close attention to how you feel daily is a key part of the monitoring process.
Daily Awareness of Side Effects
Be vigilant for any unusual symptoms. You should contact your healthcare provider immediately if you experience swelling of the hands or feet, sudden vision changes, severe headaches, or other concerning symptoms.
Tracking Blood Pressure and Fluid Retention
Estradiol can sometimes cause your body to retain fluid, which can lead to an increase in blood pressure. Monitoring both at home can provide early warnings of potential issues.
Monthly Breast Self-Exams
In addition to clinical exams and mammograms, performing a monthly breast self-exam helps you become familiar with your own body and notice any changes that should be reported to your doctor.
Understanding the Risks and Trade-offs
Comprehensive monitoring is required because estradiol, like any medication, has potential side effects and interacts with underlying health conditions. Full transparency with your doctor is non-negotiable for your safety.
The Importance of a Full Medical History
Before you even begin, your doctor must have a complete picture of your health. It is crucial to discuss any history of abnormal vaginal bleeding, blood clots, heart disease, high blood pressure, gallbladder or liver disease, certain cancers (breast, uterine, ovarian), or a history of stroke.
Medication and Supplement Interactions
Other substances can interfere with estradiol. Be sure to disclose all medications, vitamins, and supplements you take, including over-the-counter products like St. John's wort.
Proper Application and Handling
How you use the patch directly impacts its safety and effectiveness. Patches must be applied to clean, dry, hairless skin, and you should rotate application sites to prevent irritation.
Avoiding Accidental Overdose
Never apply heat sources like heating pads or electric blankets over the patch site, and avoid direct, prolonged sunlight. Heat can increase the rate of medication absorption, leading to an unintended high dose. For the same reason, patches may need to be removed before an MRI.
How to Apply This to Your Health
Your approach to monitoring should be guided by a clear understanding of the goals and potential risks of your therapy.
- If your primary focus is safety: Prioritize disclosing your complete medical history and adhering strictly to the schedule of professional exams and screenings.
- If your primary focus is effectiveness: Be diligent about proper patch application, rotating sites, and discussing any persistent symptoms with your doctor to ensure your dosage is correct.
- If your primary focus is long-term health: Commit to monthly breast self-exams and regular check-ins to continuously reassess the need for therapy, always aiming for the lowest effective dose.
Ultimately, successful monitoring transforms you into an active, informed partner in managing your own health.
Summary Table:
| Monitoring Type | Key Actions | Frequency / Notes |
|---|---|---|
| Professional Oversight | Physical exams, cancer screenings (mammogram, Pap smear), dose evaluation | Every 3-6 months |
| Self-Monitoring | Track side effects, blood pressure, breast self-exams | Daily & Monthly |
| Risk Management | Disclose full medical history, review other medications, ensure proper patch application | Ongoing |
Need a reliable supply of high-quality transdermal patches? As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we provide healthcare and pharma distributors and brands with the consistent quality essential for patient safety. Benefit from our technical expertise for custom R&D and development to create a patch that meets your specific needs. Contact our team today to discuss your requirements.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
- Capsaicin Chili Medicated Pain Relief Patches
- Mugwort Wormwood Pain Relief Patch for Neck Pain
People Also Ask
- What are pain relief patches and how are they used? A Guide to Safe, Targeted Relief
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief
- How effective are pain relief patches for muscle pain? Target Localized Pain with Transdermal Delivery
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- How should pain relief patches be applied and used? A Guide to Safe & Effective Targeted Relief













