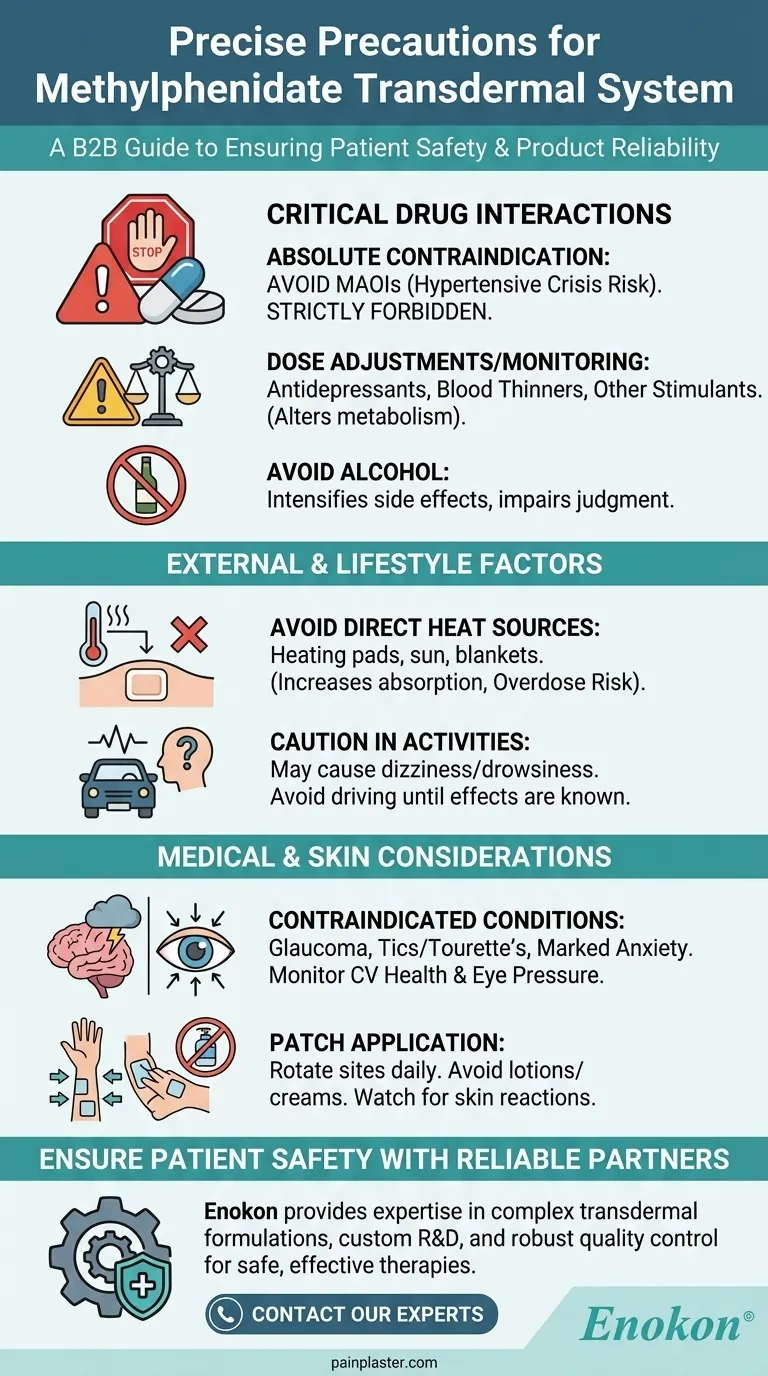
To ensure safety, the most critical precaution is to avoid using methylphenidate transdermal with Monoamine Oxidase Inhibitors (MAOIs), such as isocarboxazid or linezolid, due to the risk of a life-threatening hypertensive crisis. It is also crucial to inform your healthcare professional about all other medications you take, particularly antidepressants, blood thinners, and other stimulants, as these may require dose adjustments and careful monitoring.
The core principle of safety with the methylphenidate patch extends beyond drug interactions. It requires a comprehensive awareness of how medications, external factors like heat, and your own medical history can collectively influence the drug's absorption and effects.
Critical Drug-to-Drug Interactions
Understanding which substances interact with the methylphenidate patch is the first step toward safe use. These interactions range from absolutely forbidden combinations to those requiring careful management.
Absolute Contraindication: MAO Inhibitors
The use of methylphenidate with MAOIs (or within 14 days of discontinuing them) is strictly contraindicated.
This combination can block the normal breakdown of certain chemicals in the brain, leading to a rapid and dangerous spike in blood pressure known as a hypertensive crisis.
Medications Requiring Dose Adjustments
Many common medications do not need to be stopped but may require your doctor to adjust their dosage or monitor you more closely.
This category includes certain antidepressants, blood thinners (anticoagulants), and other CNS stimulants. Co-administration can alter how these drugs are metabolized, potentially increasing their effects or side effects.
The Impact of Alcohol
Combining alcohol with methylphenidate is generally not recommended.
Alcohol can change how the medication is absorbed and may intensify side effects like dizziness and drowsiness, impairing judgment and motor skills.
Beyond Medications: Other Critical Precautions
Effective management involves more than just monitoring other prescriptions. Environmental factors and daily activities play a significant role in how the transdermal system affects your body.
The Danger of External Heat Sources
You must avoid exposing the patch application site to any direct, external heat sources.
This includes heating pads, electric blankets, hair dryers, or prolonged sun exposure. Heat significantly increases the rate at which the medication is absorbed through the skin, creating a risk of overdose.
Impact on Physical and Mental Activities
Methylphenidate can cause dizziness, drowsiness, or changes in vision.
Until you know how the medicine affects you, you must avoid driving, operating heavy machinery, or engaging in any other potentially hazardous activities.
Skin-Related Considerations
The patch is applied directly to the skin, which requires specific care.
You should alternate the application site each day to avoid irritation. Watch for signs of contact sensitization (allergic skin reaction) or persistent loss of skin pigmentation at the application site. Do not apply lotions or creams to the area before applying the patch.
Understanding Medical Contraindications and Risks
The decision to use this medication depends heavily on your overall health profile. Certain pre-existing conditions make its use unsafe, and potential long-term risks require ongoing monitoring.
Pre-existing Psychiatric and Neurological Conditions
This medication is contraindicated for individuals with marked anxiety, tension, and agitation.
It is also not to be used in patients with motor tics or a family history or diagnosis of Tourette syndrome. For those with a history of seizures, the drug may lower the convulsive threshold.
Cardiovascular and Ocular Health
Methylphenidate can increase blood pressure and heart rate, requiring regular monitoring by your physician.
It is also contraindicated for individuals with glaucoma, as it can increase pressure within the eye.
Potential for Abuse and Dependence
As a CNS stimulant, methylphenidate has a high potential for abuse and dependence. This is a black box warning from the FDA.
Your physician will assess the risk for abuse before prescribing and will monitor for any signs of abuse or dependence throughout your therapy.
Making the Right Choice for Your Goal
Proactive communication with your healthcare provider is the foundation of safe and effective treatment. Your focus will determine your most important actions.
- If your primary focus is avoiding a severe adverse event: Immediately disclose any use of MAOIs and provide a complete list of all medications, supplements, and vitamins to your doctor.
- If your primary focus is managing daily safety: Be vigilant about avoiding heat sources near the patch, refraining from alcohol, and understanding how the medication affects your ability to drive.
- If your primary focus is long-term health: Commit to all follow-up appointments to monitor blood pressure, heart rate, skin condition, and growth in pediatric patients.
Ultimately, your safety relies on a collaborative partnership with your healthcare team to manage all factors that influence this medication's effects.
Summary Table:
| Precaution Category | Key Considerations |
|---|---|
| Critical Drug Interactions | Avoid MAOIs (risk of hypertensive crisis). Monitor with antidepressants, blood thinners, other stimulants. |
| External Factors | Avoid direct heat on patch (risk of overdose). Refrain from alcohol. Use caution when driving. |
| Medical Conditions | Contraindicated with glaucoma, marked anxiety, tics/Tourette's. Monitor cardiovascular health. |
| Application & Skin | Rotate application sites daily. Watch for skin irritation or allergic reactions. |
Ensure Patient Safety with Reliable Transdermal Delivery
As a healthcare distributor or brand, providing safe and effective transdermal solutions is paramount. Enokon is a bulk manufacturer of reliable transdermal patches and pain plasters, backed by deep technical expertise.
We partner with you to navigate complex formulations like methylphenidate, ensuring robust quality control and clear safety guidance. Benefit from our custom R&D and development services to create patches that meet precise pharmacological and safety standards.
Let's develop safer transdermal therapies together. Contact our experts today to discuss your project needs.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
- Heat Relief Capsicum Patch for Lower Back Pain Relief
- Far Infrared Heat Pain Relief Patches Transdermal Patches
People Also Ask
- How do pain relief patches compare to other pain relief methods? Discover Targeted, Long-Lasting Relief
- How should pain relief patches be applied and used? A Guide to Safe & Effective Targeted Relief
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- What are pain relief patches and how are they used? A Guide to Safe, Targeted Relief















