Properly handling a rivastigmine patch involves several key steps to ensure safety and effectiveness for both the patient and any caregivers. You must wash your hands thoroughly with soap and water immediately after applying or removing a patch. A used patch should always be folded in half with the sticky, medicated sides pressed together before it is safely discarded.
The core principle of rivastigmine patch safety extends beyond simple handling. It requires a holistic awareness of your environment, consistent communication with your healthcare providers, and a disciplined daily routine for application and disposal.
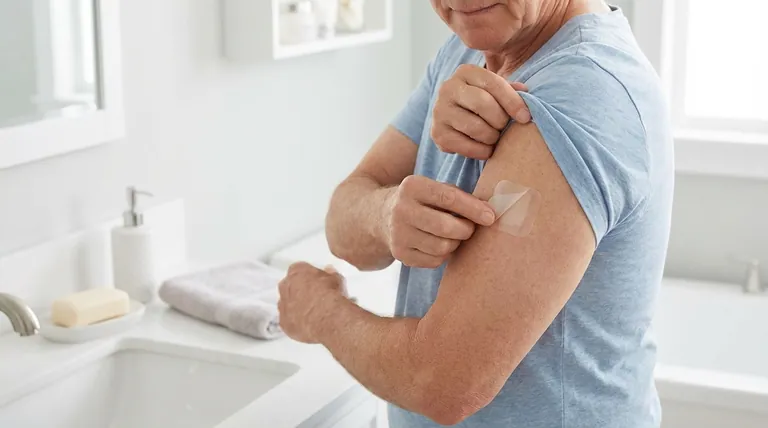
Foundational Handling and Application Safety
Correctly applying the patch is the first step in ensuring the medication works as intended while minimizing risks.
Before You Apply the Patch
Always start with clean, dry hands.
The skin where you will place the patch must be clean, dry, hairless, and free from any lotions, oils, powders, or creams. These substances can prevent the patch from sticking properly.
Ensure the skin at the application site is healthy and free from any cuts, rashes, or irritation.
After Handling the Patch
Immediately wash your hands with soap and water after applying the patch to remove any medication residue.
Be careful not to touch your eyes after handling the patch until your hands are thoroughly washed.
The Importance of Site Rotation
To prevent skin irritation, you must apply the new patch to a different spot on the skin each day.
Common application sites include the upper or lower back (where it cannot be easily removed by the patient), the upper arm, or the chest. Avoid using the same spot for at least 14 days.
Environmental and Lifestyle Precautions
Your daily activities and environment can impact how the medication is absorbed by your body.
Avoid External Heat Sources
You must avoid exposing the patch to direct, external heat sources for prolonged periods.
This includes heating pads, electric blankets, heat lamps, saunas, hot tubs, heated water beds, and extended, direct sunlight.
Excessive heat can cause the body to absorb the medication too quickly, increasing the risk of side effects.
Water Exposure and Daily Activities
You can bathe, shower, or swim while wearing the patch.
If the patch falls off, do not reapply it. Apply a new patch immediately and continue your normal schedule from there.
Activities Requiring Mental Alertness
Rivastigmine can cause dizziness or drowsiness, especially when you first start treatment or increase your dose.
Avoid driving, operating heavy machinery, or performing any other activity that requires full mental alertness until you know how the medication affects you.
Critical Medical Considerations
Open and honest communication with your entire healthcare team is essential for your safety.
Informing Your Healthcare Providers
Before starting, tell your doctor about any allergies, especially to rivastigmine or other cholinesterase inhibitors.
Disclose your complete medical history, particularly if you have asthma, ulcers, seizures, heart conditions, or liver or kidney disease. Provide a full list of all medications and supplements you take.
Before Medical Procedures
You must inform any doctor or dentist that you are using a rivastigmine patch before any surgery or medical procedure.
Crucially, the patch must be removed before you undergo a Magnetic Resonance Imaging (MRI) scan.
Pregnancy and Breastfeeding
If you are pregnant, planning to become pregnant, or are breastfeeding, you must consult your doctor before using this medication to discuss the potential risks and benefits.
Safe Disposal and Accidental Exposure
Proper disposal is a critical safety step to protect others from accidental contact with the medication.
The Correct Disposal Method
After removing a used patch, immediately fold it in half so the sticky, medicated sides press firmly together.
This seals the remaining medication inside and reduces the risk of accidental exposure.
Preventing Exposure to Others
Dispose of the folded patch in a place that is safely out of the reach of children and pets. Accidental application or ingestion by a child or pet can be extremely dangerous.
A Quick Safety Checklist for Your Routine
Use this checklist to ensure you are covering the most important safety measures.
- If you are a new user: Prioritize discussing your full medical history and all current medications with your doctor before the first application.
- For daily application and removal: Make washing your hands and rotating the application site a non-negotiable part of your routine.
- If you live an active lifestyle: Be aware that external heat sources like saunas, hot tubs, or prolonged sun exposure must be avoided while wearing the patch.
- When disposing of a used patch: Always fold it firmly in half with the sticky sides together to prevent accidental exposure to others.
Following these precautions consistently is the key to using rivastigmine safely and achieving its intended therapeutic benefit.
Summary Table:
| Precaution Category | Key Action | Why It Matters |
|---|---|---|
| Handling & Application | Wash hands before/after; apply to clean, dry, healthy skin. | Prevents improper adhesion and accidental self-exposure. |
| Site Rotation | Use a different spot each day; avoid same site for 14 days. | Reduces risk of skin irritation and ensures consistent drug delivery. |
| Environmental | Avoid direct heat sources (saunas, heating pads, prolonged sun). | Prevents overdose from rapid medication release. |
| Medical Safety | Inform doctors before MRI/surgery; discuss full medical history. | Crucial for procedural safety and managing drug interactions. |
| Disposal | Fold used patch in half (sticky sides together) before discarding. | Protects children/pets from accidental exposure to residual medication. |
Need a reliable, safely manufactured transdermal patch?
At Enokon, we are a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharmaceutical distributors and brands. Our technical expertise ensures every patch is designed with patient safety and precise drug delivery in mind.
Benefit from our custom R&D and development services to create a patch that meets your exact specifications.
Contact our experts today to discuss your transdermal patch needs.
Visual Guide

Related Products
- Herbal Eye Protection Patch Eye Patch
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- When should a doctor be consulted regarding the use of this patch? Key Safety Guidelines
- How can using eye patches contribute to a self-care skincare routine? Boost Hydration & Relaxation
- What factors should be considered when purchasing eye patches? Essential Guide for Safe & Effective Use
- What are the main benefits of using eye patches in a skincare routine? Revitalize Your Under-Eye Area
- Can under eye patches smooth fine lines and wrinkles? Hydrate & Plump for Youthful Skin













