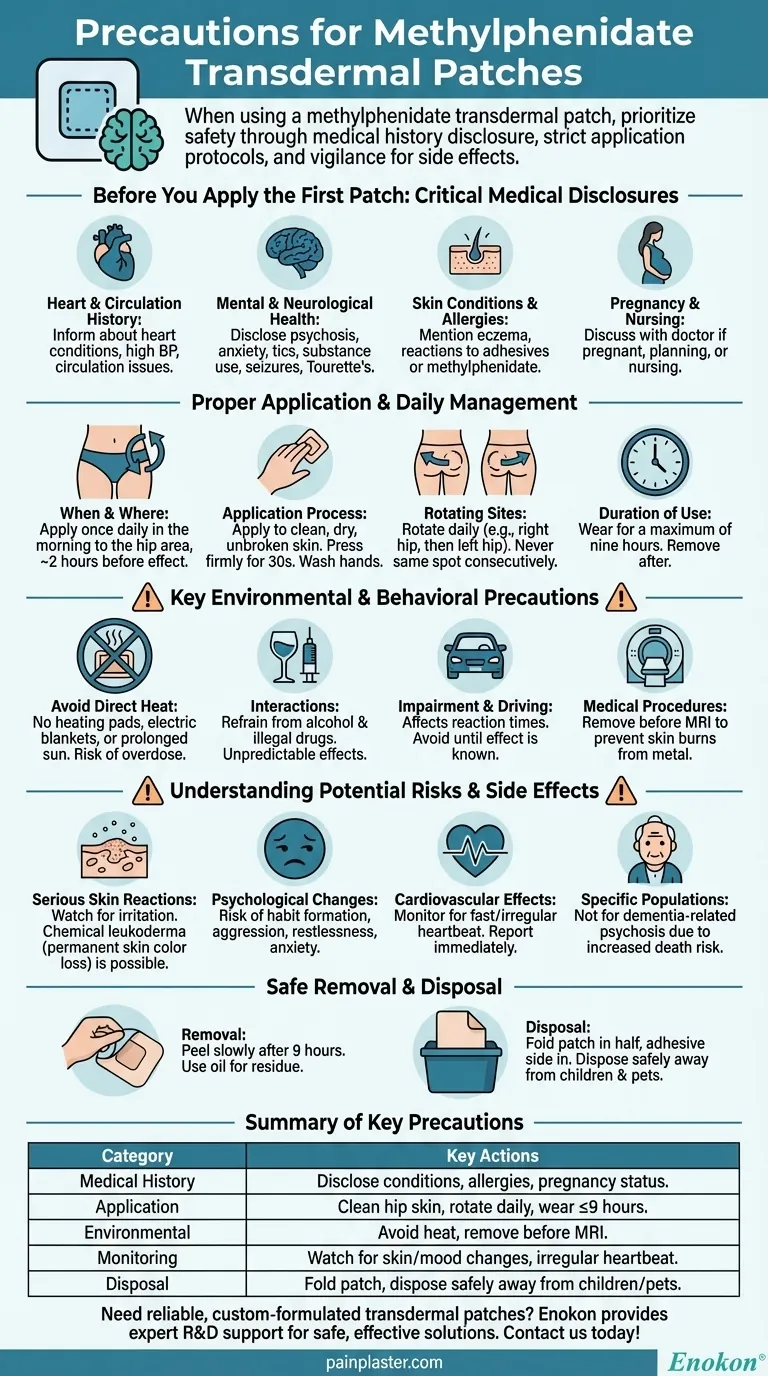
When using a methylphenidate transdermal patch, the most critical precautions involve providing a complete medical history to your doctor, adhering to strict application and removal protocols, and avoiding direct heat sources on the patch. You must also diligently monitor for skin reactions, including permanent changes in skin color, and be aware of potential psychological and cardiovascular side effects. This medication should always be part of a comprehensive treatment plan supervised by a healthcare professional.
The safe and effective use of a methylphenidate transdermal patch is not just about applying it correctly; it is about establishing a vigilant partnership with your doctor to manage both the physical application and the potential systemic effects of the medication.
Before You Apply the First Patch: Critical Medical Disclosures
Your doctor can only prescribe this medication safely with a full picture of your health. Withholding information can lead to serious adverse events.
Heart and Circulation History
You must inform your doctor of any personal or family history of heart conditions, high blood pressure, or circulation issues in your fingers and toes.
Mental and Neurological Health
Disclose any history of mental health conditions such as psychosis, anxiety, or tics. A history of substance use, seizures, or Tourette's syndrome is also critical information for your provider.
Skin Conditions and Allergies
Tell your doctor about any pre-existing skin conditions or any known allergies, especially to methylphenidate or adhesives. This helps prevent severe skin reactions.
Pregnancy and Nursing
It is crucial to inform your doctor if you are pregnant, planning to become pregnant, or nursing, as the medication may not be suitable.
Proper Application and Daily Management
Correct application is fundamental to ensuring a consistent dose and minimizing skin irritation.
When and Where to Apply
The patch should be applied once daily in the morning, approximately two hours before the therapeutic effect is needed. The recommended application site is the hip area.
The Application Process
Apply the patch to clean, dry, and unbroken skin. Press it firmly in place for about 30 seconds to ensure the edges are sealed, and wash your hands thoroughly after application.
Rotating Application Sites
To prevent skin irritation, you must rotate the application site daily. For example, if you apply it to the right hip one day, use the left hip the next. Never apply it to the same spot on consecutive days.
Duration of Use
The patch should be worn for a maximum of nine hours. Do not use more than one patch at a time unless specifically instructed by your doctor.
Key Environmental and Behavioral Precautions
Your actions and environment while wearing the patch can significantly impact its safety and efficacy.
The Danger of Direct Heat
You must avoid exposing the patch to direct heat sources like heating pads, electric blankets, or prolonged direct sunlight. Heat can increase the rate at which the medication is absorbed, leading to a potential overdose.
Interactions with Other Substances
Refrain from using alcohol or illegal drugs while on this medication. These substances can cause unpredictable and dangerous interactions.
Impairment and Driving
The medication can affect your reaction times and judgment. Avoid driving or operating heavy machinery until you know how the patch affects you.
Medical Procedures
If you are scheduled for an MRI, you must remove the patch beforehand, as it may contain metal components that could cause skin burns during the procedure.
Understanding the Potential Risks and Side Effects
While effective for many, the methylphenidate patch carries risks that require careful monitoring. This is a critical conversation to have with your healthcare provider.
Serious Skin Reactions
Watch for signs of skin irritation or an allergic reaction at the patch site. A known side effect is chemical leukoderma, a permanent loss of skin color at the application sites.
Psychological and Behavioral Changes
Overuse can lead to habit formation and dependence. Be aware of unusual changes in behavior, such as aggression, restlessness, anxiety, or thoughts of self-harm.
Cardiovascular Effects
Monitor for symptoms like a fast or irregular heartbeat. Any cardiovascular side effects should be reported to your doctor immediately.
Risk in Specific Populations
This medication should not be used in individuals with dementia-related psychosis due to an increased risk of death.
Safe Removal and Disposal
Proper disposal is a crucial safety step to prevent accidental exposure to others.
The Removal Process
After nine hours, peel the patch off slowly. If any adhesive remains, you can gently remove it with oil or a medical adhesive remover.
Why Proper Disposal is Critical
A used patch still contains active medication. Fold the patch in half so the adhesive side sticks to itself, and dispose of it in a way that is inaccessible to children or pets.
Making the Right Choice for Your Goal
Your approach to using the methylphenidate patch should be guided by a clear understanding of your treatment objectives and safety priorities.
- If your primary focus is daily safety: Adhere strictly to the 9-hour wear time, daily site rotation on the hip, and complete avoidance of direct heat on the patch.
- If your primary focus is long-term health: Maintain open communication with your doctor about any changes in mood, skin condition, or cardiovascular symptoms.
- If you are a caregiver: Ensure used patches are folded and disposed of immediately and safely to prevent accidental exposure to children or pets.
Ultimately, successful treatment relies on your commitment to following these precautions as part of a comprehensive plan managed by your healthcare team.
Summary Table:
| Precaution Category | Key Actions |
|---|---|
| Medical History | Disclose heart conditions, mental health history, skin allergies, and pregnancy status. |
| Application | Apply to clean, dry hip skin; rotate sites daily; wear for ≤9 hours. |
| Environmental | Avoid direct heat sources (e.g., heating pads); remove before MRI. |
| Monitoring | Watch for skin discoloration, mood changes, or irregular heartbeat. |
| Disposal | Fold patch to seal adhesive; dispose safely away from children/pets. |
Need reliable, custom-formulated transdermal patches for your healthcare brand or distribution network?
As Enokon, a bulk manufacturer of trusted transdermal patches and pain plasters, we provide expert R&D support to develop safe, effective solutions tailored to your needs. Let us help you deliver quality patient care—contact our team today to discuss your project!
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
- Heat Relief Capsicum Patch for Lower Back Pain Relief
- Far Infrared Heat Pain Relief Patches Transdermal Patches
People Also Ask
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- How should pain relief patches be applied and used? A Guide to Safe & Effective Targeted Relief
- How effective are pain relief patches for muscle pain? Target Localized Pain with Transdermal Delivery
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief

















