The Buprenorphine Transdermal Patch requires careful handling due to its potent opioid nature and specific application method. Key precautions include avoiding heat exposure, monitoring for respiratory depression, and preventing misuse. Patients must follow strict application guidelines, rotate sites weekly, and avoid concurrent use with CNS depressants. Special populations (pregnant women, children, those with liver disease) need extra caution. Regular medical supervision is essential to manage risks like addiction and accidental overdose.
Key Points Explained:
-
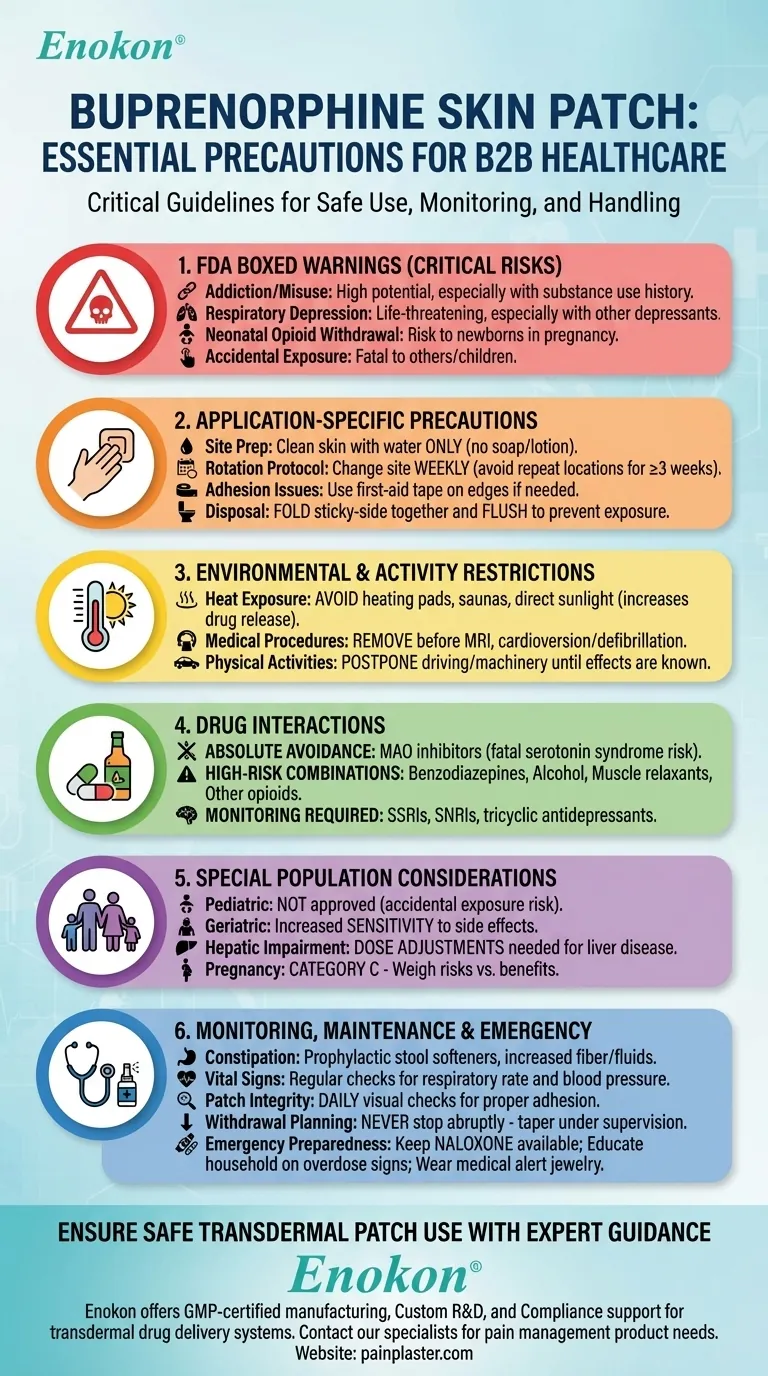
FDA Boxed Warnings (Critical Risks)
- Addiction/Misuse: High potential for abuse, especially in patients with substance use history
- Respiratory Depression: Life-threatening slowed breathing, particularly when combined with other depressants
- Neonatal Opioid Withdrawal: Risk of severe withdrawal in newborns if used during pregnancy
- Accidental Exposure: Can be fatal if patch is ingested or applied to others (especially children)
-
Application-Specific Precautions
- Site Preparation: Clean skin with water only (no soap/lotion) to ensure adhesion
- Rotation Protocol: Change application sites weekly (avoid repeating locations for ≥3 weeks)
- Adhesion Issues: Use first-aid tape on edges if needed - never cover with airtight dressings unless directed
- Disposal: Fold used patches sticky-side together and flush to prevent accidental exposure
-
Environmental & Activity Restrictions
-
Heat Exposure: Avoid:
- Heating pads/electric blankets
- Hot baths/saunas
- Direct sunlight on application site (increases drug release by up to 120%)
-
Medical Procedures: Remove before:
- MRI (risk of burns from metal in patch)
- Cardioversion/defibrillation
- Physical Activities: Postpone driving/operating machinery until effects are known
-
Heat Exposure: Avoid:
-
Drug Interactions
- Absolute Avoidance: MAO inhibitors (can cause fatal serotonin syndrome)
-
High-Risk Combinations:
- Benzodiazepines
- Alcohol
- Muscle relaxants
- Other opioids
- Monitoring Required: With SSRIs, SNRIs, and tricyclic antidepressants
-
Special Population Considerations
- Pediatric: Not approved for children (accidental exposure risk)
- Geriatric: Increased sensitivity to side effects
- Hepatic Impairment: Dose adjustments needed for liver disease
- Pregnancy: Category C - weigh risks of NOWS vs. pain management
-
Monitoring & Maintenance
- Constipation Management: Prophylactic stool softeners + increased fiber/fluids
- Vital Signs: Regular checks for respiratory rate and blood pressure
- Patch Integrity: Daily visual checks for proper adhesion
- Withdrawal Planning: Never stop abruptly - taper under medical supervision
-
Emergency Preparedness
- Keep naloxone available for overdose reversal
- Educate household members on recognizing overdose signs
- Wear medical alert jewelry indicating opioid use
The patch's convenience belies its risks - while providing steady pain relief, it demands vigilant handling akin to handling sensitive laboratory equipment where precision and protocol adherence prevent catastrophic outcomes. Have you considered creating a checklist for patients to ensure all precautions are followed systematically?
Summary Table:
| Precaution Category | Key Actions |
|---|---|
| FDA Boxed Warnings | Monitor for addiction, respiratory depression, neonatal withdrawal, accidental exposure |
| Application Protocol | Clean skin with water, rotate sites weekly, secure edges with tape, fold & flush used patches |
| Environmental Restrictions | Avoid heat sources/MRI, remove for medical procedures, delay driving until effects known |
| Drug Interactions | Avoid MAO inhibitors, CNS depressants, alcohol; monitor with antidepressants |
| Special Populations | Adjust doses for liver disease, avoid in children, caution in elderly/pregnant |
| Emergency Preparedness | Keep naloxone accessible, educate household members, wear medical alert jewelry |
Ensure Safe Transdermal Patch Use with Expert Guidance
For healthcare distributors and brands requiring reliable transdermal drug delivery systems, Enokon offers:
- GMP-certified manufacturing of medical patches with precise dosing
- Custom R&D for specialized formulations (including abuse-deterrent features)
- Compliance support for FDA/EMA regulatory requirements
Contact our transdermal technology specialists today to discuss your pain management product needs.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Knee Pain Patch Heat Patches for Pain Relief
- Far Infrared Pain Patch Relief Pain Reliever for Back
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- What are pain relief patches and how are they used? A Guide to Safe, Targeted Relief
- How effective are pain relief patches for muscle pain? Target Localized Pain with Transdermal Delivery
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism