Oxybutynin is well-suited for transdermal delivery due to its molecular properties, including moderate molecular weight (357 daltons), lipophilicity, and weak base characteristics that favor skin permeation. The Oxybutynin Transdermal Patch leverages these properties to provide controlled, consistent drug delivery while minimizing systemic side effects like dry mouth. Its matrix design ensures stable absorption across multiple application sites (abdomen, buttock, hip) over 3-4 days, making it a practical option for overactive bladder treatment.
Key Points Explained:
-
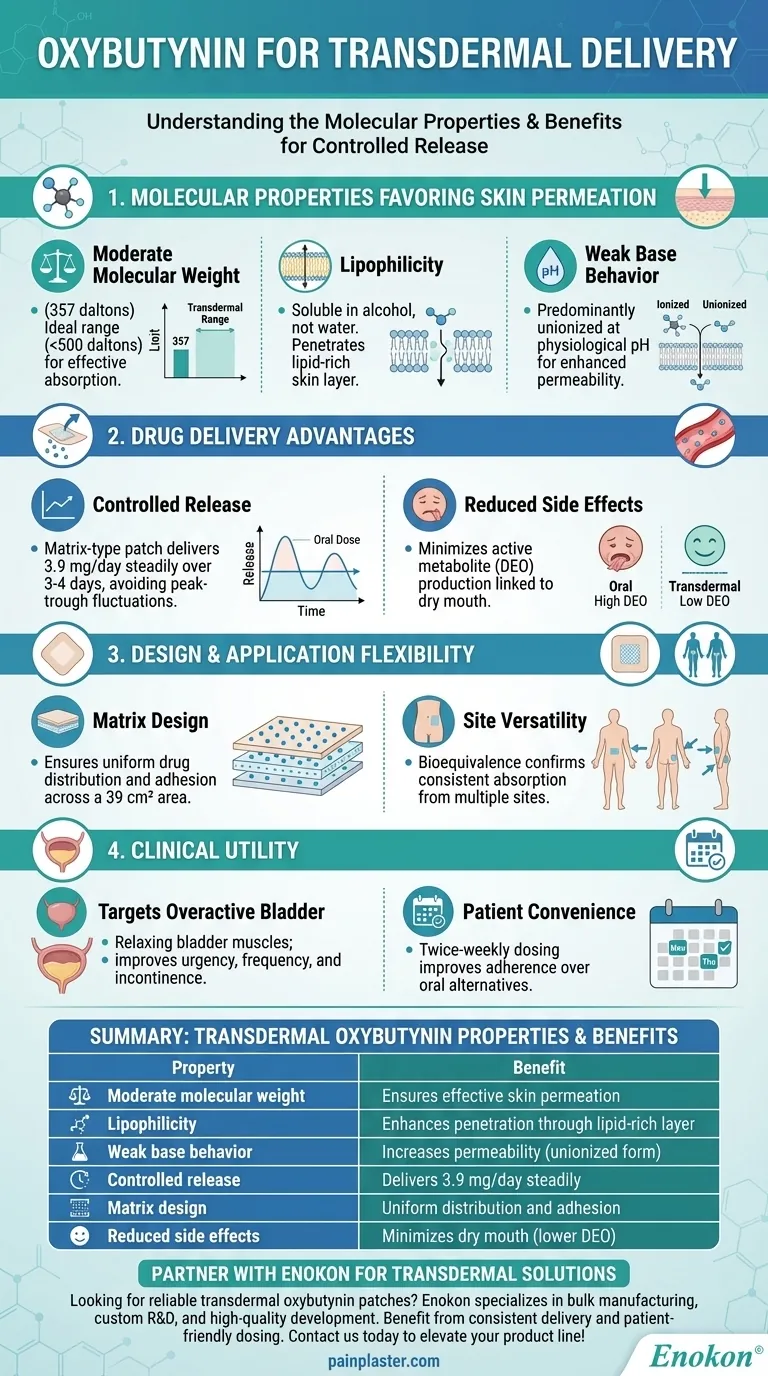
Molecular Properties Favoring Skin Permeation
- Moderate molecular weight (357 daltons): Falls within the ideal range (<500 daltons) for transdermal absorption.
- Lipophilicity: Soluble in alcohol but not water, enabling penetration through the skin's lipid-rich outer layer.
- Weak base behavior: Predominantly unionized at physiological pH, enhancing permeability compared to ionized forms.
-
Drug Delivery Advantages
- Controlled release: The matrix-type patch delivers 3.9 mg/day steadily over 3-4 days, avoiding peak-trough plasma fluctuations.
- Reduced side effects: Minimizes production of DEO (active metabolite linked to dry mouth) compared to oral formulations.
-
Design and Application Flexibility
- Matrix design: Ensures uniform drug distribution and adhesion across a 39 cm² patch area.
- Site versatility: Bioequivalence studies confirm consistent absorption whether applied to the abdomen, buttock, or hip.
-
Clinical Utility
- Targets overactive bladder by relaxing bladder muscles, improving urgency, frequency, and incontinence.
- Offers patient convenience with twice-weekly dosing, improving adherence over oral alternatives.
These properties collectively make oxybutynin a model candidate for transdermal delivery, balancing efficacy with tolerability.
Summary Table:
| Property | Benefit |
|---|---|
| Moderate molecular weight | Ensures effective skin permeation (<500 daltons) |
| Lipophilicity | Enhances penetration through the skin's lipid-rich outer layer |
| Weak base behavior | Increases permeability by remaining unionized at physiological pH |
| Controlled release | Delivers 3.9 mg/day steadily, avoiding peak-trough fluctuations |
| Matrix design | Provides uniform drug distribution and adhesion across application sites |
| Reduced side effects | Minimizes dry mouth by lowering DEO metabolite production |
Looking for reliable transdermal oxybutynin patches for your healthcare or pharmaceutical brand? Enokon specializes in bulk manufacturing of high-quality transdermal patches and pain plasters, backed by technical expertise in custom R&D and development. Benefit from consistent drug delivery, reduced side effects, and patient-friendly dosing. Contact us today to discuss your requirements and elevate your product line!
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
People Also Ask
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
















