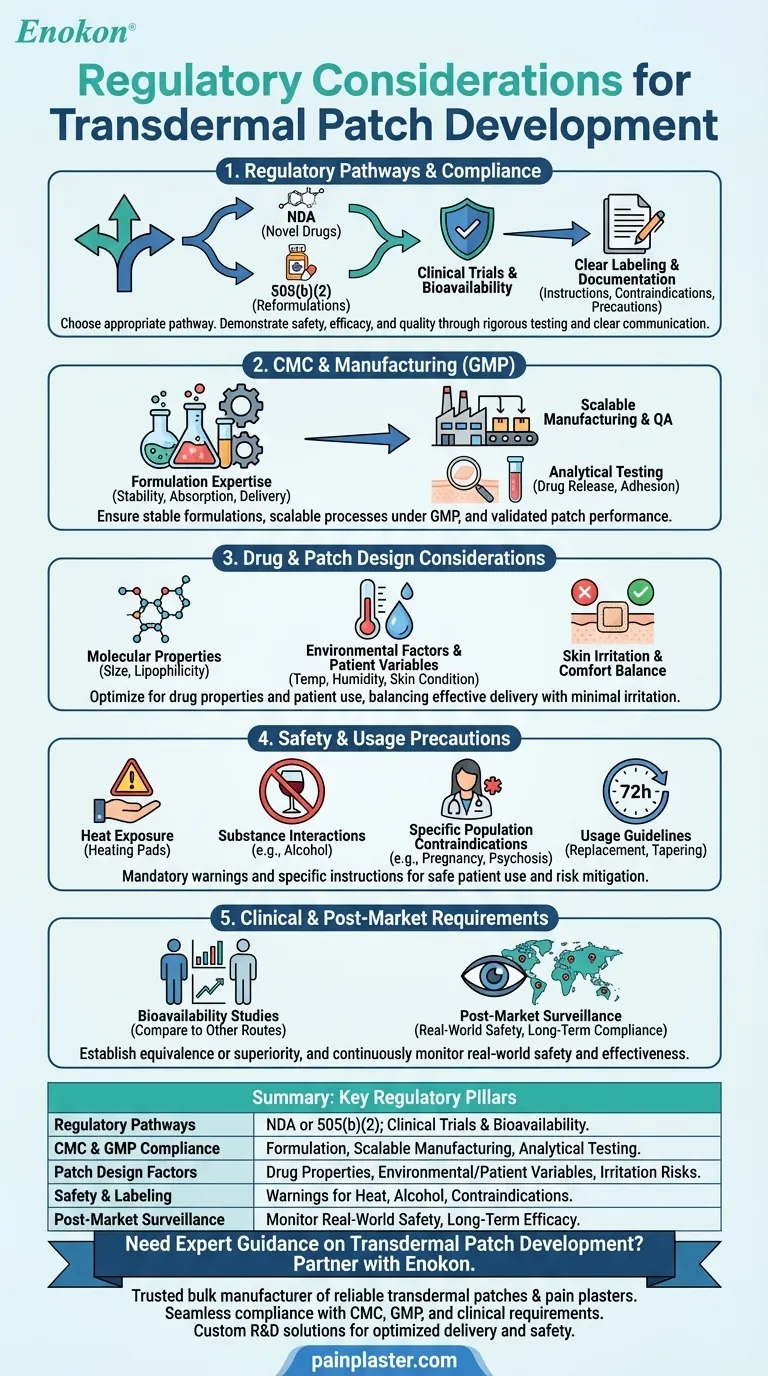
Transdermal patch development involves navigating a complex regulatory landscape to ensure safety, efficacy, and quality. Key considerations include compliance with chemistry, manufacturing, and controls (CMC) requirements, adherence to Good Manufacturing Practices (GMP), and thorough clinical testing for bioavailability and safety. Patch design must account for drug properties (e.g., molecular size, polarity) and environmental factors, while labeling and documentation must meet strict standards. Regulatory pathways like NDAs or 505(b)(2) may apply, depending on the drug's novelty. Precautions such as skin irritation risks and usage restrictions (e.g., avoiding heat exposure) must also be addressed to gain approval and ensure patient safety.

Key Points Explained:
-
Regulatory Pathways and Compliance
- Sponsors must choose between a New Drug Application (NDA) for novel drugs or a 505(b)(2) pathway for reformulations of approved drugs.
- Compliance with transdermal patch regulations requires demonstrating safety, efficacy, and quality through clinical trials, including bioavailability studies.
- Clear labeling and documentation are mandatory, detailing usage instructions, contraindications (e.g., dementia-related psychosis), and precautions (e.g., avoiding alcohol or heat exposure).
-
Chemistry, Manufacturing, and Controls (CMC)
- Formulation expertise is critical to ensure drug stability, absorption, and consistent delivery rates.
- Scalability and quality assurance during commercial manufacturing must align with GMP standards.
- Analytical testing validates patch performance, including drug release profiles and adhesion properties.
-
Drug and Patch Design Considerations
- Molecular properties (e.g., small size, lipophilicity) influence absorption rates and must be optimized during development.
- Environmental factors (temperature, humidity) and patient-specific variables (skin condition, age) affect efficacy and require testing under realistic conditions.
- Patches must balance drug delivery with comfort, minimizing skin irritation or allergic reactions.
-
Safety and Usage Precautions
- Labeling must warn against risks like skin irritation, heat exposure (e.g., heating pads), and interactions with substances like alcohol.
- Specific populations (pregnant/nursing individuals, those with psychosis) may require contraindications.
- Usage guidelines (e.g., replacement every 72 hours, tapering protocols) must be clearly communicated to patients.
-
Clinical and Post-Market Requirements
- Bioavailability studies compare transdermal delivery to other routes (e.g., oral) to establish equivalence or superiority.
- Post-market surveillance monitors real-world safety, ensuring long-term compliance and addressing unforeseen adverse effects.
By addressing these areas, developers can navigate regulatory hurdles while delivering effective, patient-friendly transdermal therapies. Have you considered how patch adhesives might evolve to reduce skin irritation without compromising drug delivery?
Summary Table:
| Key Regulatory Consideration | Details |
|---|---|
| Regulatory Pathways | NDA for novel drugs; 505(b)(2) for reformulations. Requires clinical trials and bioavailability studies. |
| CMC & GMP Compliance | Formulation stability, scalable manufacturing, and analytical testing (drug release, adhesion). |
| Patch Design Factors | Optimize drug properties (size, lipophilicity), environmental/patient variables, and skin irritation risks. |
| Safety & Labeling | Warn against heat exposure, alcohol interactions, and contraindications for high-risk populations. |
| Post-Market Surveillance | Monitor real-world safety and long-term efficacy post-approval. |
Need expert guidance on transdermal patch development? Partner with Enokon, a trusted bulk manufacturer of reliable transdermal patches and pain plasters for healthcare brands and distributors. Our technical expertise ensures seamless compliance with CMC, GMP, and clinical testing requirements, while our custom R&D solutions optimize drug delivery and patient safety. Contact us today to discuss your project!
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Herbal Eye Protection Patch Eye Patch
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- What is the purpose of using high-flatness precision casting surfaces? Ensure Precise Dosing in Transdermal Patches
- What was the conclusion of the study regarding the transdermal patches? Promising Results for Dental Pain Relief
- Why Use a Desiccator with AlCl3 or CaCl2 for Patch Moisture Testing? Achieve Maximum Stability & Performance
- Why is high-precision UV-Vis spectrophotometry used for transdermal drug delivery? Master Your Permeation Data
- Why is an ultrasonic cleaner necessary before solution casting of Upadacitinib patches? Ensure Matrix Quality
- Who can use diclofenac gel? Safe Pain Relief for Arthritis & Injuries
- What are topical pain medicines and how are they applied? Discover Targeted Pain Relief Solutions
- Why is multi-point measurement using a high-precision thickness gauge required for transdermal patches? Ensure Dosage.

















