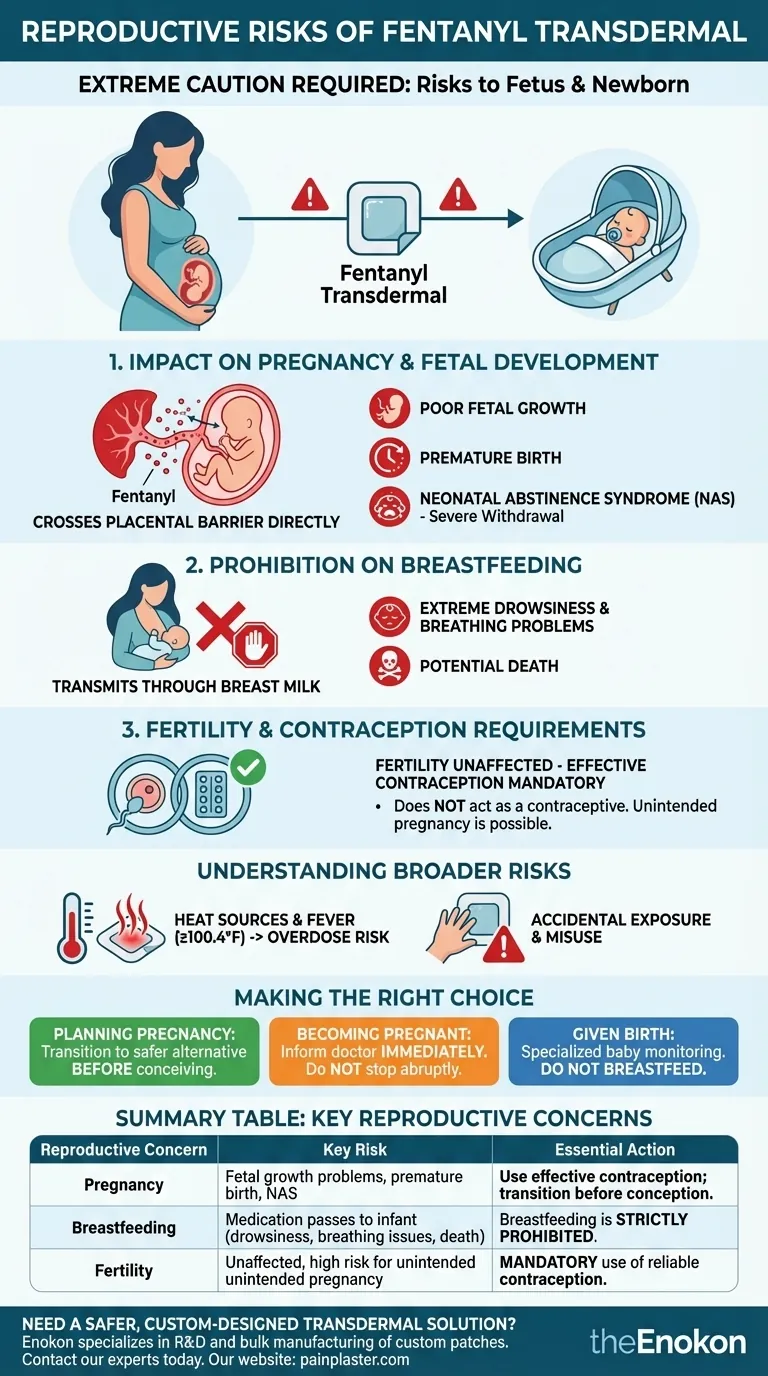
Using a fentanyl transdermal patch during your reproductive years requires extreme caution. This medication poses significant risks to a developing fetus and a newborn infant. Fentanyl can cause fetal growth problems, premature birth, and severe withdrawal symptoms in a baby after birth. For this reason, breastfeeding is strictly prohibited, and effective contraception is mandatory during treatment.
The core issue is that fentanyl crosses the placental barrier and is transmitted through breast milk. This direct exposure presents serious dangers to a fetus and a nursing infant, making pregnancy and breastfeeding incompatible with treatment.

The Impact on Pregnancy and Fetal Development
When you use a fentanyl patch during pregnancy, the medication does not stay with you. Its chemical properties allow it to pass from your bloodstream directly to the fetus, leading to significant complications.
Risks of In-Utero Exposure
Direct exposure to fentanyl can impair fetal development. The most documented risks include poor fetal growth and an increased likelihood of premature birth.
Neonatal Abstinence Syndrome (NAS)
A baby exposed to fentanyl throughout pregnancy can become physically dependent on the opioid. After birth, the supply is cut off, leading to a painful and dangerous condition known as neonatal withdrawal or Neonatal Abstinence Syndrome (NAS).
This requires intensive medical monitoring and treatment for the newborn.
The Mechanism of Transmission
Fentanyl is a small, fat-soluble (lipid-soluble) molecule. This structure allows it to easily pass through the biological barrier of the placenta, which is designed to protect the fetus.
Fertility and Contraception Requirements
It is a critical misunderstanding to assume that a powerful medication like fentanyl impacts fertility. This is not the case, which creates a high-risk situation if not managed properly.
Fertility is Unaffected
The fentanyl patch does not act as a contraceptive. Your ability to become pregnant remains unchanged while using it, meaning an unintended pregnancy is entirely possible.
The Mandate for Effective Contraception
Given the severe risks to a fetus, using a reliable and effective form of contraception is required throughout the entire course of treatment with the fentanyl patch.
The Prohibition on Breastfeeding
The same properties that allow fentanyl to cross the placenta also allow it to enter breast milk, posing a direct threat to a nursing infant.
Transmission Through Breast Milk
Fentanyl has been shown to be present in the milk of mothers using the medication. A nursing infant would ingest the opioid with every feeding.
Dangers for the Nursing Infant
For a newborn, exposure to fentanyl through breast milk can cause extreme drowsiness, severe breathing problems, and potentially death. Because of this, breastfeeding is absolutely prohibited while using the fentanyl patch.
Understanding the Broader Safety Risks
The reproductive concerns exist within a larger context of risk associated with this potent opioid. Understanding these helps frame the importance of careful management, especially during pregnancy.
Risk of Overdose from Increased Absorption
Your body temperature can change how quickly you absorb fentanyl from the patch. Heat sources like heating pads or hot baths, excessive exercise, or a fever (100.4°F/38°C or higher) can cause a dangerous surge of medication into your system, leading to overdose. This is a critical concern during a potential pregnancy, where fevers or the use of hot water for comfort are common.
Accidental Exposure and Misuse
Fentanyl patches are dangerous to anyone who comes into contact with them, especially children. Even used patches contain enough medication to be fatal. Following prescribed schedules (typically changing every 72 hours) without deviation is essential to prevent overdose.
Making the Right Choice for Your Goal
Navigating pain management with fentanyl requires a proactive and honest partnership with your healthcare provider. Your specific life stage dictates the necessary conversation.
- If you are planning a pregnancy: You must work with your doctor to create a plan to transition to a safer pain management alternative well before you begin trying to conceive.
- If you become pregnant while using the patch: Inform your doctor immediately. Do not stop the medication abruptly, but work with them to develop the safest possible plan for you and your baby.
- If you are of reproductive age: You must use a reliable form of contraception consistently throughout your treatment to prevent an unintended and high-risk pregnancy.
- If you have given birth while on fentanyl: Your baby will require specialized medical monitoring for withdrawal symptoms, and you absolutely must not breastfeed.
Ultimately, open communication with your healthcare team is the most critical tool for ensuring your safety and the health of a potential child.
Summary Table:
| Reproductive Concern | Key Risk | Essential Action |
|---|---|---|
| Pregnancy | Fetal growth problems, premature birth, Neonatal Abstinence Syndrome (NAS) | Use effective contraception; transition to safer medication before conception. |
| Breastfeeding | Medication passes to infant, causing severe drowsiness, breathing issues, or death | Breastfeeding is strictly prohibited. |
| Fertility | Fertility is unaffected, creating high risk for unintended pregnancy | Mandatory use of reliable contraception throughout treatment. |
Need a Safer, Custom-Designed Transdermal Solution?
If you are a healthcare or pharmaceutical distributor or brand seeking reliable, high-quality transdermal patches, Enokon can help. We specialize in the custom R&D and bulk manufacturing of transdermal patches and pain plasters.
- Benefit from our technical expertise to develop formulations tailored to specific patient needs and safety profiles.
Let's collaborate to create advanced transdermal delivery systems that prioritize patient safety.
Contact our experts today to discuss your custom development project.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Heat Pain Relief Patches Transdermal Patches
People Also Ask
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief
- How effective are pain relief patches for muscle pain? Target Localized Pain with Transdermal Delivery
- How do pain relief patches provide targeted relief? Discover the Science Behind Effective Pain Management
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief














