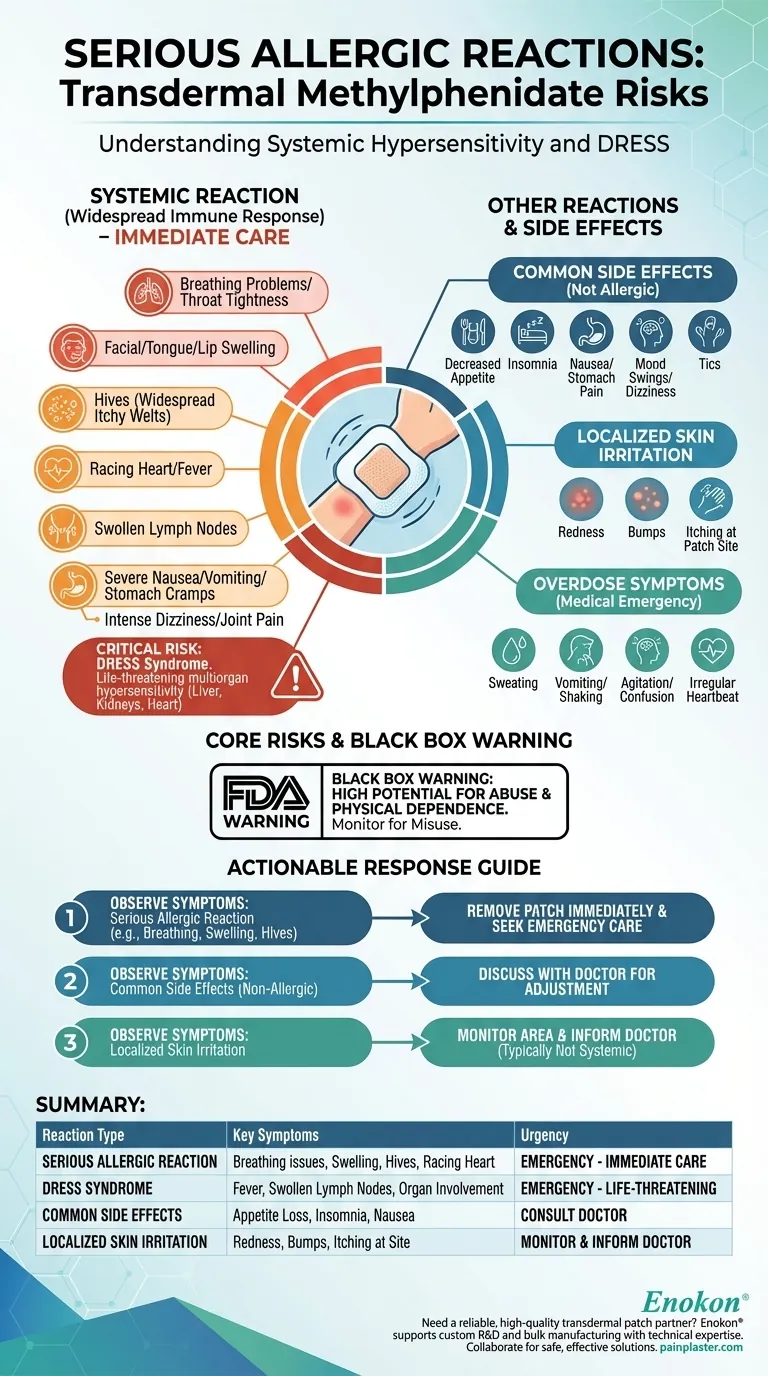
Serious allergic reactions to transdermal methylphenidate can manifest as a systemic response, involving symptoms that go far beyond simple skin irritation. These may include difficulty breathing, facial or throat swelling, a racing heart, hives, fever, and swollen lymph nodes, all of which require immediate medical attention.
While many users experience localized skin irritation, the most critical risk is a systemic hypersensitivity reaction. This can escalate into a rare but life-threatening condition known as DRESS, which affects internal organs and requires urgent medical intervention.
Recognizing a Systemic Allergic Reaction
A true allergic reaction to transdermal methylphenidate involves the entire body's immune system, not just the skin where the patch is applied. It is crucial to identify these symptoms promptly.
Symptoms Requiring Immediate Medical Attention
The signs of a serious allergic reaction signal a widespread immune response. These symptoms include:
- Breathing problems or tightness in the throat
- Swelling of the face, tongue, or lips
- Hives (raised, itchy welts on the skin)
- Racing heart or fever
- Swollen lymph nodes
- Severe nausea, vomiting, or stomach cramps
- Intense dizziness or joint pain
Any of these symptoms warrants seeking emergency medical help.
DRESS Syndrome: A Critical Multiorgan Reaction
A particularly severe, though rare, allergic reaction is DRESS (Drug Reaction with Eosinophilia and Systemic Symptoms).
This is a multiorgan hypersensitivity reaction that can be life-threatening. It goes beyond typical allergy symptoms and can cause damage to vital organs like the liver, kidneys, and heart.
Distinguishing Reactions from Common Side Effects
To respond appropriately, it is essential to differentiate a dangerous allergic reaction from more common, less severe side effects or symptoms of overdose.
Common Side Effects
These effects are not allergic in nature but are common with methylphenidate. They include:
- Decreased appetite and weight loss
- Trouble sleeping (insomnia)
- Nausea, vomiting, or stomach pain
- Sudden mood swings or dizziness
- Tics (sudden, repetitive movements or sounds)
Localized Skin Irritation
Many users will experience redness, bumps, or itching at the application site. This is typically a localized skin irritation and is not considered a systemic allergic reaction unless it is accompanied by hives or other symptoms elsewhere on the body.
Overdose Symptoms
An overdose is a distinct medical emergency and is not an allergic reaction. Symptoms include:
- Sweating and facial redness
- Vomiting or shaking
- Agitation, confusion, or hallucinations
- Irregular heartbeat
Suspected overdose requires immediate emergency medical attention.
Understanding the Core Risks
While effective, transdermal methylphenidate carries risks that must be understood and monitored by both the patient and the prescribing doctor.
The Black Box Warning
The FDA requires this medication to have a black box warning, its most serious type. This warning highlights the high potential for abuse and physical dependence.
Patients should be assessed for risk before starting therapy and monitored for signs of misuse throughout treatment.
How to Respond to a Suspected Reaction
Your response should be based on the specific symptoms you or someone else is experiencing.
- If you observe signs of a serious allergic reaction (e.g., facial swelling, hives, difficulty breathing): Remove the patch immediately and seek emergency medical care without delay.
- If you experience common but non-allergic side effects (e.g., appetite loss, sleep issues): Discuss these with your prescribing doctor to determine if an adjustment is needed.
- If you notice only localized skin irritation at the patch site: Monitor the area and inform your doctor, but recognize this is typically not a dangerous systemic reaction.
Understanding these critical distinctions is the key to using this medication safely and effectively.
Summary Table:
| Reaction Type | Key Symptoms | Urgency |
|---|---|---|
| Serious Allergic Reaction | Difficulty breathing, facial/throat swelling, hives, racing heart | Emergency - Immediate Care Required |
| DRESS Syndrome | Fever, swollen lymph nodes, organ involvement (liver, kidneys) | Emergency - Life-Threatening |
| Common Side Effects | Decreased appetite, insomnia, nausea, mood swings | Consult Prescribing Doctor |
| Localized Skin Irritation | Redness, bumps, itching at patch site | Monitor & Inform Doctor |
Need a reliable, high-quality transdermal patch partner?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we understand the critical importance of safety and precise formulation. Our technical expertise ensures consistent quality and supports custom R&D for healthcare and pharma distributors and brands.
Let's collaborate to develop safe and effective transdermal solutions. Contact our experts today to discuss your requirements.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Capsaicin Chili Medicated Pain Relief Patches
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief
- What are pain relief patches and how are they used? A Guide to Safe, Targeted Relief
- How should pain relief patches be applied and used? A Guide to Safe & Effective Targeted Relief
- How do pain relief patches provide targeted relief? Discover the Science Behind Effective Pain Management
- How effective are pain relief patches for muscle pain? Target Localized Pain with Transdermal Delivery














