Before using a Testosterone Transdermal Patch, it's crucial to evaluate both its benefits and potential risks through a comprehensive assessment of individual health factors. Key considerations include allergies, age-specific risks (pediatric/geriatric), breastfeeding status, drug interactions, and pre-existing medical conditions. Women who are or may become pregnant must avoid contact due to fetal risks. Regular medical monitoring is essential to detect side effects like prostate cancer risk, cardiovascular issues, or metabolic changes. Special precautions are needed for MRI procedures (patch removal required) and surgical planning. Underlying conditions such as diabetes, sleep apnea, or clotting disorders may require adjusted treatment approaches.
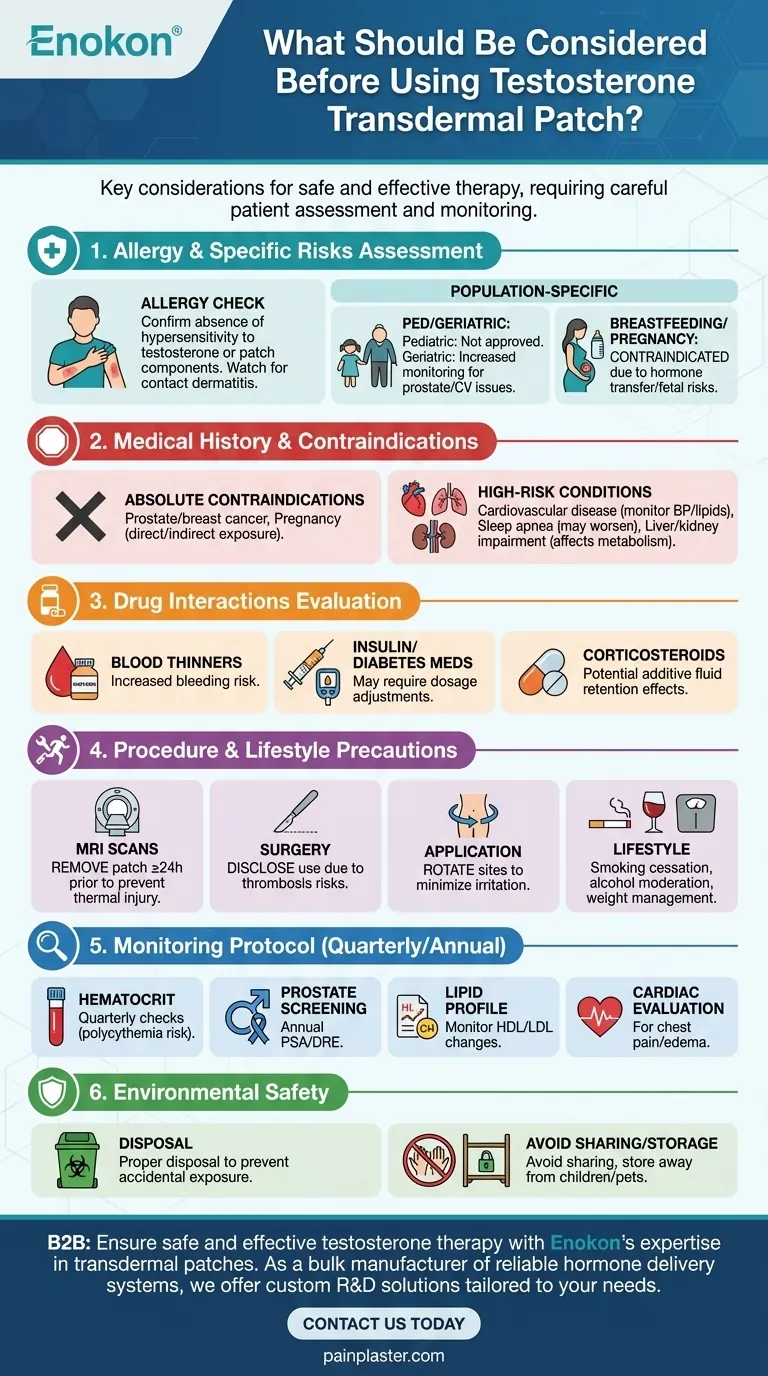
Key Points Explained:
-
Allergy Assessment
- Confirm absence of hypersensitivity to testosterone or patch components
- Watch for signs of contact dermatitis at application sites
-
Population-Specific Risks
- Pediatric: Not approved for children due to developmental risks
- Geriatric: Increased monitoring for prostate/cardiovascular issues
- Breastfeeding: Contraindicated due to hormone transfer risks
-
Drug Interaction Evaluation
- Blood thinners (warfarin): Increased bleeding risk
- Insulin/diabetes medications: May require dosage adjustments
- Corticosteroids: Potential additive fluid retention effects
-
Medical History Review
-
Absolute contraindications:
- Prostate/breast cancer
- Pregnancy (direct/indirect exposure)
-
High-risk conditions:
- Cardiovascular disease (monitor BP/lipids)
- Sleep apnea (may worsen symptoms)
- Liver/kidney impairment (affects metabolism)
-
Absolute contraindications:
-
Procedure Precautions
- MRI scans: Remove patch ≥24h prior to prevent thermal injury
- Surgery: Disclose use due to thrombosis risks
- Application sites: Rotate to minimize skin irritation
-
Lifestyle Considerations
- Smoking cessation advised (synergistic CVD risk)
- Alcohol moderation (hepatic stress)
- Weight management (obesity affects hormone metabolism)
-
Monitoring Protocol
- Quarterly hematocrit checks (polycythemia risk)
- Annual prostate screening (PSA/DRE)
- Lipid profile monitoring (HDL/LDL changes)
- Cardiac evaluation for chest pain/edema
-
Environmental Safety
- Proper disposal to prevent accidental exposure
- Avoid sharing patches (dosing errors)
- Storage away from children/pets
The patch offers convenient testosterone delivery but requires careful patient selection and ongoing supervision. Have you considered how application site rotation might affect steady-state hormone levels? This underscores the balance between therapeutic benefits and individualized risk management in hormone replacement therapy.
Summary Table:
| Key Consideration | Details |
|---|---|
| Allergies | Check for hypersensitivity to testosterone or patch components. |
| Age-Specific Risks | Pediatric: Not for children. Geriatric: Monitor prostate/cardiovascular health. |
| Drug Interactions | Blood thinners, diabetes meds, and corticosteroids may require adjustments. |
| Medical History | Avoid if prostate/breast cancer or pregnancy. High-risk: CVD, sleep apnea, liver/kidney issues. |
| Procedure Precautions | Remove before MRI scans. Disclose use before surgery. Rotate application sites. |
| Monitoring | Regular checks for hematocrit, prostate health, lipids, and cardiac evaluation. |
Ensure safe and effective testosterone therapy with Enokon's expertise in transdermal patches. As a bulk manufacturer of reliable hormone delivery systems, we offer custom R&D solutions tailored to your needs. Contact us today to discuss how our patches can support your healthcare or pharma distribution goals with precision and safety.
Visual Guide
Related Products
- Prostate Pain Kidney Health Care Patch for Men
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Medical Cooling Gel Patches for Fever Cooling Patches
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- What lifestyle factors should be considered when choosing between testosterone patches and injections? Find Your Best Fit
- What should be done before undergoing an MRI while using testosterone patches? Remove it to prevent serious burns.
- What should patients tell their doctor before using testosterone patches? A Guide to Safe Treatment
- What should be done if a dose of testosterone patches is missed? Regain Stability and Safety
- What should be done in case of a testosterone patch overdose? A Step-by-Step Emergency Guide
















