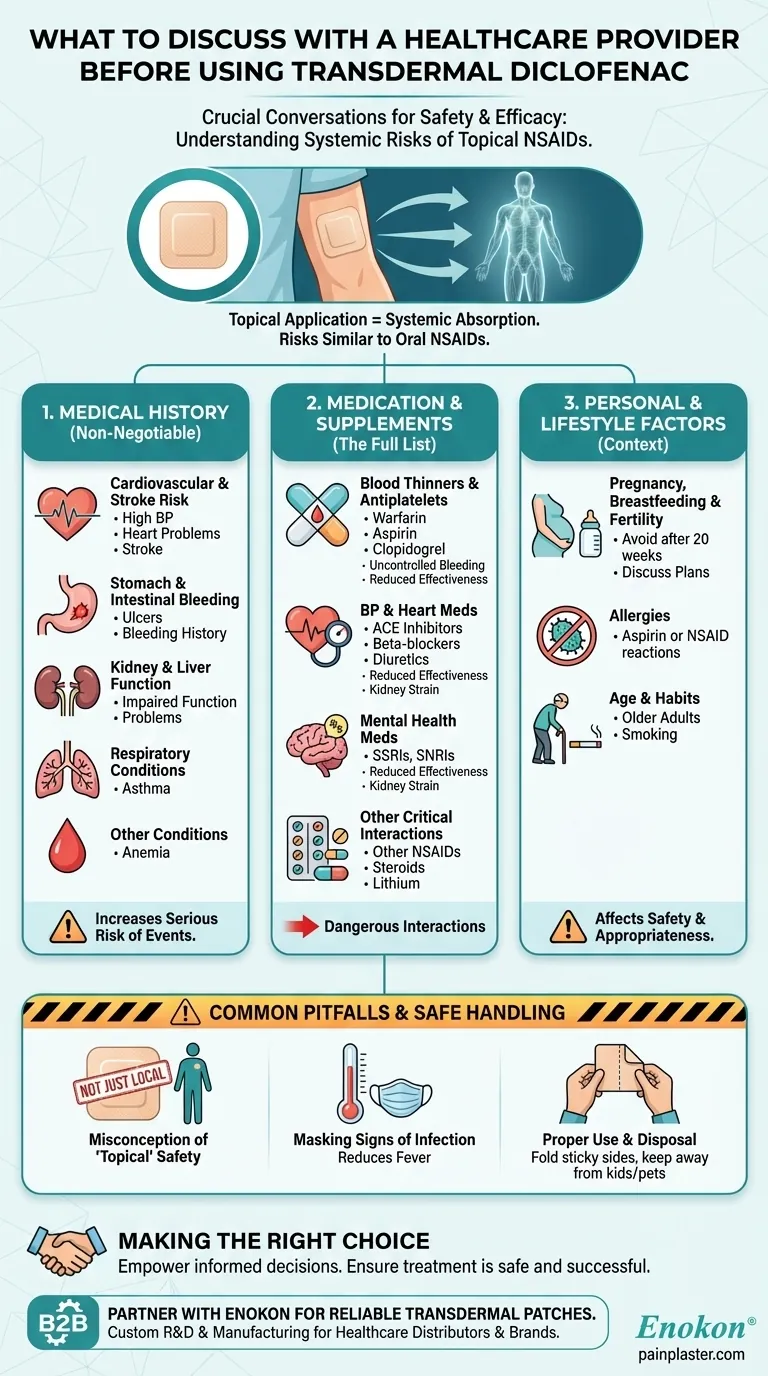
Before using transdermal diclofenac, you must have a comprehensive discussion with your healthcare provider about your complete medical history, all medications and supplements you take, and key lifestyle factors. This conversation is critical because even though the medication is applied to the skin, it is absorbed into your bloodstream and can cause serious systemic side effects.
The core principle to understand is that topical diclofenac carries many of the same significant risks as oral NSAIDs, including potential harm to your heart, stomach, and kidneys. Open communication with your provider is the primary tool to ensure your safety.

Why a Detailed Medical History is Non-Negotiable
A thorough review of your past and present health conditions is the first step in determining if transdermal diclofenac is a safe option for you.
Cardiovascular and Stroke Risk
You must disclose any history of high blood pressure, heart problems (like a previous heart attack), or stroke. NSAIDs, including diclofenac, can increase the risk of serious cardiovascular events.
Stomach and Intestinal Bleeding
Inform your provider about any history of stomach ulcers or bleeding. Even in patch form, diclofenac can increase the risk of dangerous gastrointestinal bleeding, a risk that is much higher for those with a prior history.
Kidney and Liver Function
Discuss any known liver or kidney problems. These organs are responsible for processing and clearing the medication from your body. Impaired function can lead to a toxic buildup of the drug.
Respiratory Conditions
It is essential to mention if you have asthma. Some individuals with asthma are sensitive to NSAIDs, which can trigger severe breathing difficulties.
Other Key Conditions
Be sure to also discuss other conditions like anemia, as NSAIDs can contribute to bleeding and worsen this condition.
Revealing Your Complete Medication and Supplement List
Diclofenac can interact dangerously with many common prescriptions, over-the-counter drugs, and supplements. Your provider needs a complete list to prevent harmful interactions.
Blood Thinners and Antiplatelets
Combining diclofenac with blood thinners (e.g., warfarin) or antiplatelet medicines (e.g., clopidogrel, aspirin) significantly elevates your risk of uncontrolled bleeding.
Blood Pressure and Heart Medications
Diclofenac can reduce the effectiveness of many medications for high blood pressure, including ACE inhibitors (e.g., lisinopril), ARBs (e.g., losartan), beta-blockers (e.g., metoprolol), and diuretics ("water pills"). This combination can also put significant strain on your kidneys.
Mental Health Medications
If you take antidepressants like SSRIs (e.g., fluoxetine) or SNRIs (e.g., venlafaxine), combining them with diclofenac increases the risk of bleeding.
Other Critical Interactions
Your provider must also know if you use other NSAIDs (like ibuprofen or naproxen), corticosteroids (e.g., prednisone), lithium, methotrexate, digoxin, or cyclosporine, as these can lead to serious adverse effects when taken with diclofenac.
Understanding the Personal and Lifestyle Factors
Your personal circumstances and habits play a crucial role in the safety and appropriateness of this medication.
Pregnancy, Breastfeeding, and Fertility
Discuss if you are pregnant, planning a pregnancy, or breastfeeding. Diclofenac should be avoided around or after 20 weeks of pregnancy unless specifically directed by a doctor. You should also mention any concerns about fertility.
Allergies
Clearly state any known allergies, especially a previous reaction to aspirin or any other NSAID. An allergic reaction can be severe.
Age and Habits
Mention if you are an older adult, as this group is often at a higher risk for side effects. Disclosing habits like smoking is also important, as it can compound cardiovascular risks.
Common Pitfalls and Safe Handling
Understanding the risks and proper use of the patch itself is a key part of the conversation.
The Misconception of "Topical" Safety
Discuss your understanding that this is not just a local treatment. The drug enters your system and can affect your entire body.
Masking Signs of Infection
Be aware that diclofenac can reduce fever, potentially masking the early signs of an infection.
Proper Use and Disposal
Confirm you understand the instructions for use: do not wear the patch while bathing or showering, keep it away from children and pets, and always fold a used patch with the sticky sides together before disposal.
Making the Right Choice for Your Goal
This information empowers you and your provider to make the safest decision for your specific health profile.
- If you have a history of heart or blood pressure issues: Your primary concern is ensuring this medication won't counteract your current treatment or increase your risk of a cardiovascular event.
- If you have a history of stomach ulcers or GI bleeding: Your discussion must focus on whether the potential for pain relief outweighs the significant risk of a new, potentially life-threatening bleed.
- If you take multiple medications: Your goal is to have your provider and pharmacist conduct a thorough review to prevent a harmful drug interaction, particularly one affecting your kidneys or bleeding risk.
- If you are pregnant, planning to be, or breastfeeding: The conversation should center on safer alternatives, as diclofenac is generally not recommended in these situations, especially in later pregnancy.
Being an active and informed partner in your healthcare is the most effective way to ensure your treatment is both safe and successful.
Summary Table:
| Topic to Discuss | Key Details to Mention | Why It's Critical |
|---|---|---|
| Medical History | Heart conditions, stroke, stomach ulcers, liver/kidney issues, asthma. | Increases risk of serious cardiovascular, GI, and other systemic side effects. |
| Current Medications | Blood thinners, blood pressure meds, SSRIs/SNRIs, other NSAIDs, corticosteroids. | High risk of dangerous interactions, including bleeding and kidney damage. |
| Lifestyle & Personal Factors | Pregnancy/breastfeeding plans, age, smoking habits, known allergies to NSAIDs. | Affects drug safety and appropriateness; diclofenac is not recommended in later pregnancy. |
Need a reliable, high-quality transdermal patch for your pain management portfolio?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we partner with healthcare and pharmaceutical distributors and brands. We provide the technical expertise for custom R&D and development, ensuring your products meet the highest standards of safety and efficacy for end-users.
Contact our experts today to discuss how we can support your product development needs.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Mugwort Wormwood Pain Relief Patch for Neck Pain
People Also Ask
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- How should pain relief patches be applied and used? A Guide to Safe & Effective Targeted Relief
- How do pain relief patches compare to other pain relief methods? Discover Targeted, Long-Lasting Relief
- How do pain relief patches provide targeted relief? Discover the Science Behind Effective Pain Management
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief

















