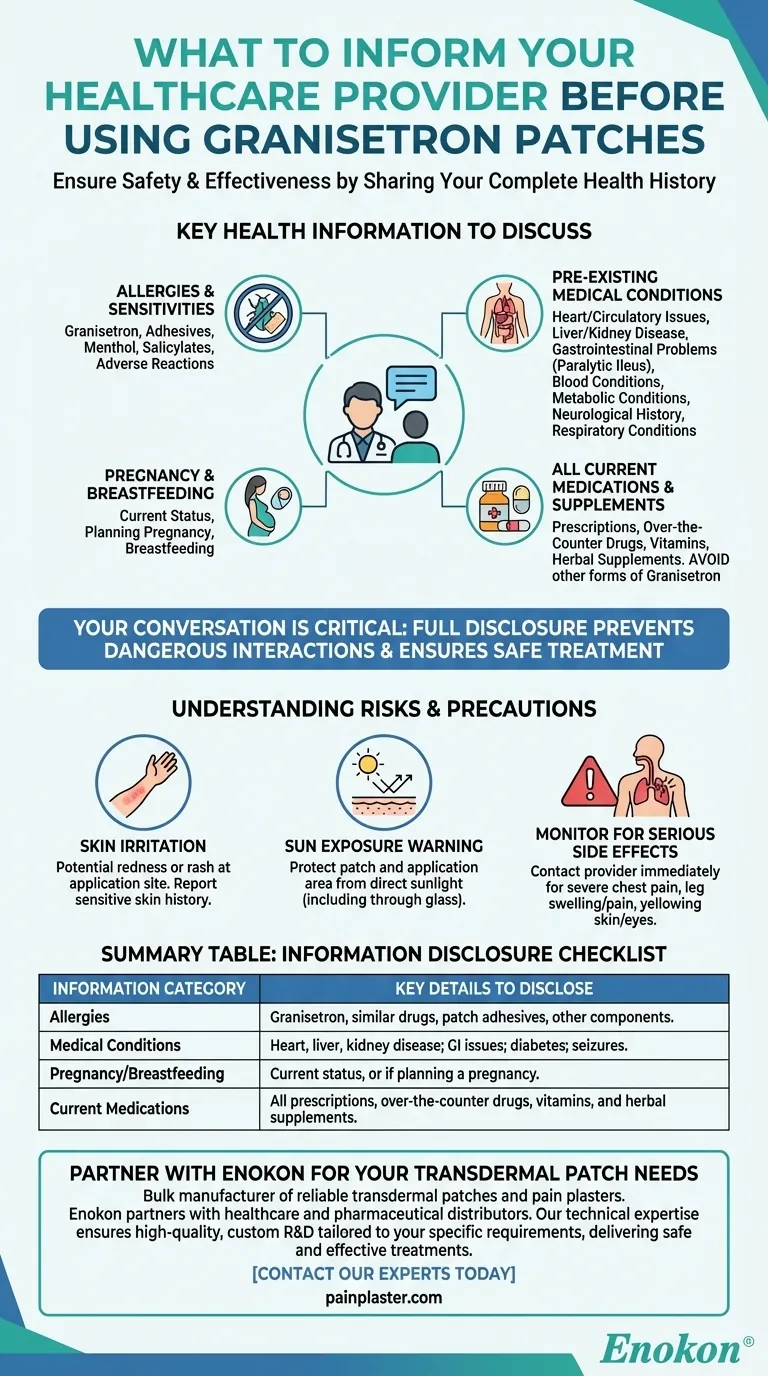
Before you apply a granisetron patch, you must provide your healthcare provider with a complete picture of your health. This includes any history of liver or heart problems, any known allergies to granisetron or patch adhesives, and whether you are pregnant, trying to become pregnant, or breastfeeding. Disclosing all current medications and pre-existing conditions is essential for your safety.
Your conversation with your healthcare provider is the most critical step in ensuring your treatment is both safe and effective. Full transparency about your health history prevents dangerous interactions and allows your doctor to confirm that a granisetron patch is the right choice for you.

Why Full Disclosure Is a Critical Safety Measure
A granisetron patch is designed to prevent nausea and vomiting, often from chemotherapy, by releasing medication through your skin. However, your body is a complex system, and pre-existing conditions or other medications can change how granisetron works or increase the risk of side effects.
Providing a complete medical history allows your doctor to anticipate potential issues, adjust your treatment plan, and ensure the medication works as intended without causing harm.
Key Health Information to Discuss
Organize your health information before your appointment to ensure you cover every important detail. Focus on these core areas.
Allergies and Sensitivities
You must report any known allergies, not just to medications. Be specific about any history of adverse reactions to granisetron or similar anti-nausea drugs. Also mention sensitivities to patch components like adhesives, menthol, or salicylates (including aspirin).
Pre-existing Medical Conditions
This is the most crucial part of the conversation. Be sure to mention if you have or have ever had:
- Heart and Circulatory Issues: This includes any heart problems, a history of stroke, high or low blood pressure, or high cholesterol.
- Liver or Kidney Disease: Proper organ function is critical for processing the medication.
- Gastrointestinal Problems: Specifically mention if you have had a condition called paralytic ileus, where intestinal movement stops.
- Blood-related Conditions: Inform your doctor about a history of low white blood cell counts.
- Metabolic Conditions: This includes diabetes, high blood sugar, or high prolactin levels.
- Neurological History: A history of seizures must be discussed.
- Respiratory Conditions: Mention any breathing problems, sleep disorders, or asthma.
Pregnancy and Breastfeeding
Your provider must know if you are pregnant, planning to become pregnant, or currently breastfeeding. This information is vital for protecting both you and your child, as the medication can be transferred.
All Current Medications and Supplements
Provide a complete list of everything you take. This includes all prescriptions, over-the-counter drugs, vitamins, and herbal supplements. Taking other forms of granisetron (tablets, liquid) at the same time as the patch is not recommended and should be discussed.
Understanding the Risks and Precautions
Using a transdermal patch comes with unique considerations that you should be aware of before starting treatment.
The Risk of Skin Irritation
The adhesives and medication in the patch can cause skin irritation, redness, or a rash at the application site. Inform your doctor if you have sensitive skin or have reacted to patches or medical tape in the past.
Sun Exposure Warning
The area of skin where the patch is applied, and the patch itself, must be protected from direct sunlight. This includes sunlight through glass, so keep the area covered with clothing.
Monitoring for Serious Side Effects
While using the patch, you still need to be aware of your body. Contact your provider immediately if you experience symptoms like severe chest pain, pain or swelling in your legs, or yellowing of the skin or eyes (jaundice), as these could indicate more serious issues.
Making the Right Choice for Your Health
Use your appointment to form a partnership with your provider. Being prepared helps them give you the best possible care.
- If you have a complex medical history: Write down a list of your conditions and current medications before your appointment to ensure you don't forget anything.
- If you have known skin sensitivities: Ask your doctor about the signs of a patch reaction and what you should do if one occurs.
- If you are planning a family: Discuss the implications of this treatment on pregnancy or breastfeeding well in advance.
Open communication with your healthcare provider is your most powerful tool for a safe and successful treatment.
Summary Table:
| Information Category | Key Details to Disclose |
|---|---|
| Allergies | Granisetron, similar drugs, patch adhesives, or other components. |
| Medical Conditions | Heart, liver, or kidney disease; gastrointestinal issues; diabetes; seizures. |
| Pregnancy/Breastfeeding | Current status, or if planning a pregnancy. |
| Current Medications | All prescriptions, over-the-counter drugs, vitamins, and herbal supplements. |
Partner with Enokon for Your Transdermal Patch Needs
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon partners with healthcare and pharmaceutical distributors and brands. Our technical expertise ensures high-quality, custom R&D and development tailored to your specific requirements, helping you deliver safe and effective treatments to patients.
Contact our experts today to discuss how we can support your product development.
Visual Guide
Related Products
- Herbal Eye Protection Patch Eye Patch
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- How can using eye patches contribute to a self-care skincare routine? Boost Hydration & Relaxation
- When should a doctor be consulted regarding the use of this patch? Key Safety Guidelines
- What factors should be considered when purchasing eye patches? Essential Guide for Safe & Effective Use
- How quickly can you see results from using under eye patches? Instant Brightening & Long-Term Benefits
- How do eye patches enhance the effectiveness of eye creams? Boost Your Eye Care Routine















