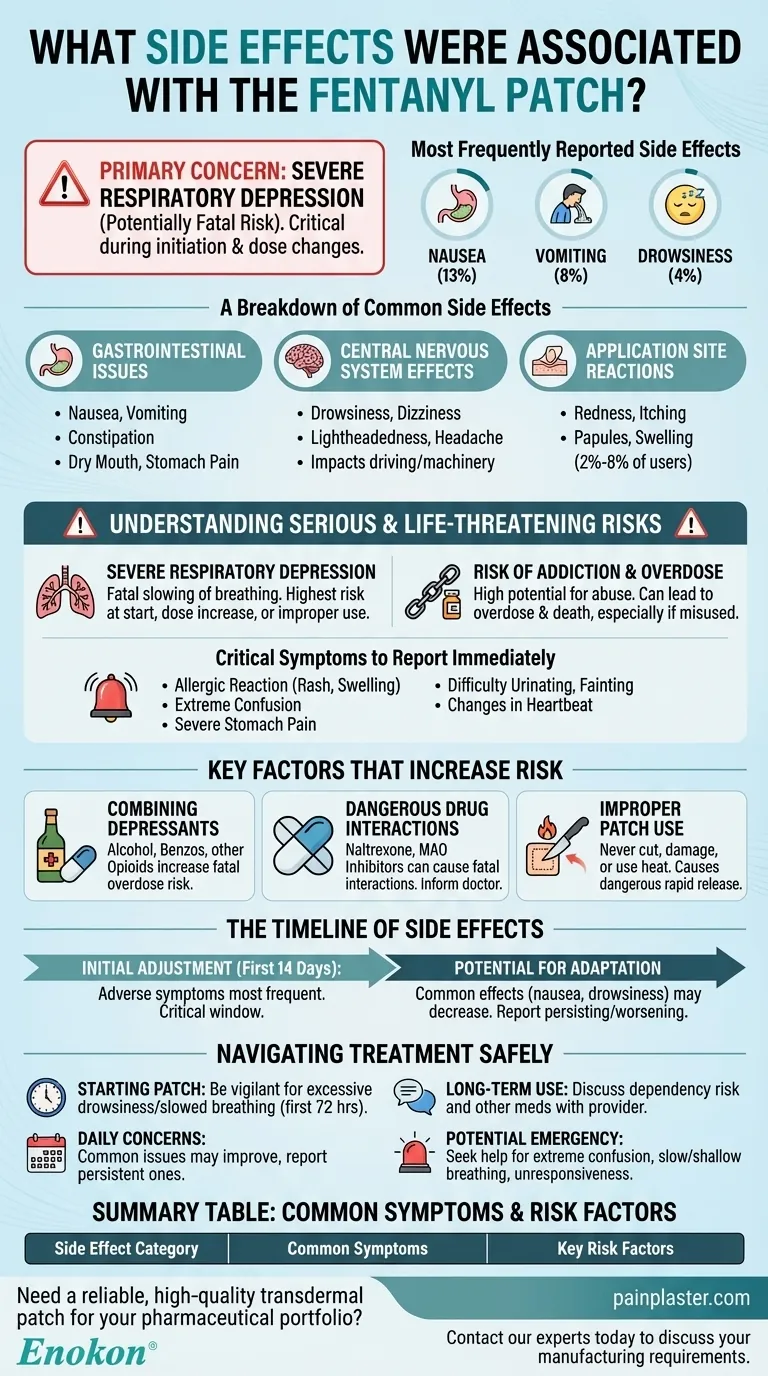
The most frequently reported side effects of the fentanyl patch are gastrointestinal and central nervous system issues, such as nausea, vomiting, and drowsiness. Clinical data shows specific rates of nausea (13%), vomiting (8%), and drowsiness (4%), with other common effects including constipation, dizziness, headache, and mild skin irritation at the application site.
While many side effects of the fentanyl patch are manageable and may lessen over time, the primary concern is the risk of severe, potentially fatal respiratory depression. Understanding this risk, especially when starting the medication or changing doses, is critical for safe use.

A Breakdown of Common Side Effects
While serious risks demand attention, understanding the common, less severe side effects is a practical first step for anyone using the patch.
Gastrointestinal Issues
Nausea and vomiting are among the most cited side effects. Constipation, a common issue with opioids, is also frequently reported, alongside dry mouth and potential stomach pain.
Central Nervous System Effects
Drowsiness, dizziness, lightheadedness, and headache are very common. These effects can impact your ability to drive or operate machinery and require significant caution.
Application Site Reactions
The skin where the patch is applied can sometimes react. While most patients (over 78%) show no irritation, a small percentage (2%-8%) may experience itching, redness, papules, or swelling at the site.
Understanding the Serious and Life-Threatening Risks
Beyond the more common side effects, the fentanyl patch carries significant risks that require immediate medical attention if they occur.
Severe Respiratory Depression
This is the most dangerous risk associated with fentanyl. It involves severe, possibly fatal, slowing of breathing. This risk is highest when first starting the medication, after a dose increase, or if the patch is used improperly.
Risk of Addiction and Overdose
Fentanyl is a powerful opioid with a high potential for abuse and addiction. This can lead to overdose and death, especially if used improperly or combined with other substances.
Other Critical Symptoms to Report
You must report certain symptoms to your healthcare provider immediately. These include signs of an allergic reaction (rash, swelling), extreme confusion, severe stomach pain, difficulty urinating, fainting, or changes in your heartbeat.
Key Factors That Increase Risk
The safety of the fentanyl patch depends heavily on proper use and awareness of how it interacts with other substances.
Combining with Other Depressants
Using the fentanyl patch with alcohol or other drugs that cause drowsiness or breathing problems (like benzodiazepines or other opioids) dramatically increases the risk of severe side effects, including fatal overdose.
Dangerous Drug Interactions
Fentanyl can interact negatively with other medications. Certain pain medications (opioid antagonists like naltrexone), and especially MAO inhibitors, can cause a serious or fatal drug interaction. Always inform your doctor of every medication you are taking.
Improper Patch Use
Never cut, damage, or use heat on a fentanyl patch. Doing so can cause the medication to release too quickly, delivering a dangerously high dose that can lead to overdose.
The Timeline of Side Effects
Understanding when side effects are most likely to occur can help manage expectations and monitor for problems.
The Initial Adjustment Period
Adverse symptoms are most frequent during the first 14 days of use. This is the critical window when your body is adjusting to the medication.
Potential for Adaptation
For many users, some of the more common side effects like nausea and drowsiness may decrease over time as their body gets used to the consistent dose. However, if any side effect persists or worsens, it should be reported.
Navigating Treatment Safely
Your approach to using the patch should be guided by your specific situation and a clear understanding of the risks.
- If you are just starting the patch: Be extremely vigilant for excessive drowsiness and slowed breathing, especially in the first 72 hours and after any dose increase.
- If you are concerned about daily side effects: Know that issues like nausea and dizziness are common initially and may improve, but report any that persist or worsen to your doctor.
- If you are managing long-term use: Be constantly aware of the risk of dependency and maintain an open conversation with your provider about all other medications and substances you use.
- If you witness a potential emergency: Immediately seek help for signs like extreme confusion, very slow or shallow breathing, or unresponsiveness, as this could indicate an overdose.
Proactive communication with your healthcare provider is the most effective tool for managing side effects and ensuring the safe use of this powerful medication.
Summary Table:
| Side Effect Category | Common Symptoms | Key Risk Factors |
|---|---|---|
| Gastrointestinal | Nausea (13%), Vomiting (8%), Constipation | Dose initiation, individual sensitivity |
| Central Nervous System | Drowsiness (4%), Dizziness, Headache | Combining with other depressants (alcohol, benzos) |
| Application Site | Redness, Itching (2%-8% of users) | Skin sensitivity, improper application |
| Serious/Life-Threatening | Severe respiratory depression, addiction, overdose | Improper use (cutting patch), drug interactions (MAOIs) |
Need a reliable, high-quality transdermal patch for your pharmaceutical portfolio?
As a bulk manufacturer of safe and effective transdermal drug delivery systems, Enokon provides healthcare and pharma distributors with trusted, GMP-compliant pain plasters and custom patches. Benefit from our technical expertise for your custom R&D and development needs, ensuring patient safety and product efficacy.
Contact our experts today to discuss your manufacturing requirements.
Visual Guide
Related Products
- Icy Hot Menthol Medicine Pain Relief Patch
- Menthol Gel Pain Relief Patch
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Prostate Pain Kidney Health Care Patch for Men
People Also Ask
- What are common side effects of menthol patch? Key Risks & Safety Tips
- How should a menthol patch be applied? Follow These Steps for Safe & Effective Pain Relief
- How does menthol in the patch work to relieve pain? Discover the Science Behind Fast-Acting Relief
- How does menthol function as a topical analgesic? The Science Behind Cooling Pain Relief
- How does menthol work in the Reliever Patch? Dual-Action Pain Relief Explained

















