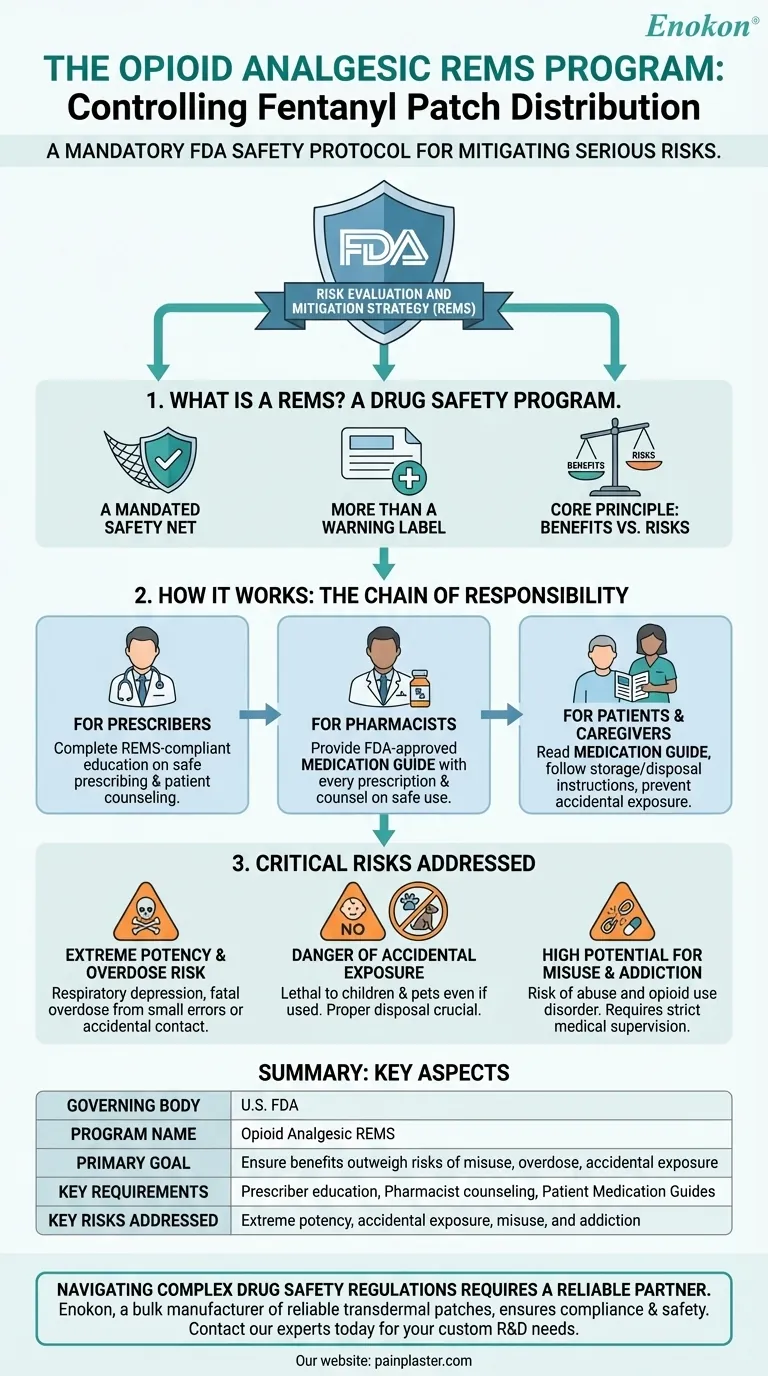
The distribution of fentanyl patches is strictly controlled by a mandatory U.S. Food and Drug Administration (FDA) safety protocol known as the Opioid Analgesic REMS (Risk Evaluation and Mitigation Strategy) program. This is not a typical prescription process but a restricted system designed to mitigate the serious risks associated with this potent medication.
The Opioid Analgesic REMS program exists to ensure that the benefits of using a powerful pain medication like fentanyl outweigh its life-threatening risks. Its primary goal is to educate prescribers, pharmacists, and patients on safe use, storage, and disposal to prevent addiction, overdose, and accidental exposure.
What is a Risk Evaluation and Mitigation Strategy (REMS)?
A REMS is a drug safety program that the FDA can require for certain medications with serious safety concerns. It is a step beyond standard warning labels or medication guides.
A Mandated Safety Net
The FDA determines that a drug's risks are significant enough to warrant specialized, ongoing management after it has been approved for the market.
More Than a Warning Label
A REMS program enforces specific actions and provides educational materials for healthcare providers and patients. This ensures everyone involved is aware of how to manage the medication's risks.
The Core Principle: Benefits vs. Risks
The ultimate goal of any REMS program is to create a system where the known benefits of using a high-risk medication can be realized while the potential for harm is actively minimized.
How the Opioid Analgesic REMS Works in Practice
The Opioid Analgesic REMS focuses heavily on education for every person who comes into contact with the medication. It establishes a chain of responsibility from the prescriber to the patient.
For Prescribers
Healthcare providers who prescribe opioid analgesics are strongly urged to complete REMS-compliant education. This training covers safe prescribing practices, pain management principles, and how to counsel patients on the specific risks of these drugs.
For Pharmacists
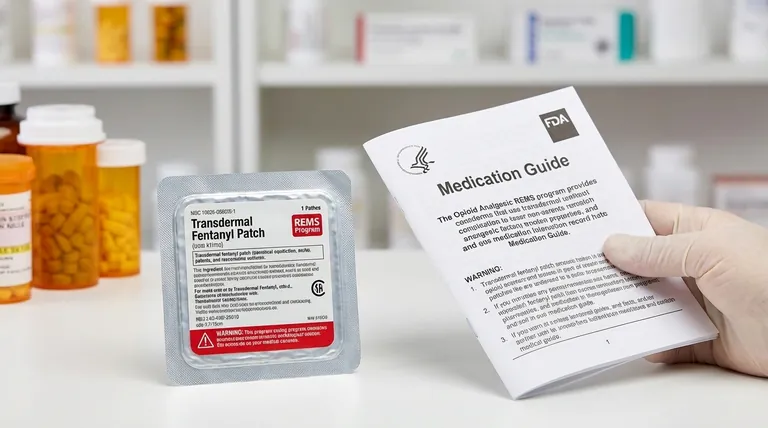
Pharmacists have a critical role. With every fentanyl patch prescription they dispense, they must provide the patient with a specific, FDA-approved Medication Guide. They are also responsible for counseling the patient on safe use and the risks outlined in the guide.
For Patients and Caregivers
Patients receive the Medication Guide, which details in plain language how to apply, store, and dispose of fentanyl patches safely. It explains the signs of overdose and the extreme danger of accidental exposure, especially to children.
Understanding the Critical Risks Addressed by REMS
The strict controls of the REMS program are in direct response to the well-documented and severe dangers of fentanyl.
Extreme Potency and Overdose Risk
Fentanyl is a powerful synthetic opioid. Even a small error in dosage or accidental contact with the gel from a patch can lead to a fatal overdose by causing respiratory depression, which is the slowing or stopping of breathing.
Danger of Accidental Exposure
A used fentanyl patch still contains a significant amount of medication. If a child or pet touches or ingests a used patch, it can be lethal. The REMS program emphasizes the critical importance of proper disposal, which often involves folding the patch in half with the sticky sides together and flushing it down the toilet.
High Potential for Misuse and Addiction
Like all opioids, fentanyl carries a high risk of misuse, abuse, and the development of opioid use disorder. The REMS program aims to ensure it is only used under strict medical supervision for appropriate conditions.
Key Takeaways for Safe Handling
Navigating a REMS-controlled medication requires diligence from everyone involved. Understanding your role is the most important step toward safety.
- If you are a patient or caregiver: Read the Medication Guide thoroughly, follow all storage and disposal instructions precisely, and never allow anyone else to use the medication.
- If you are a prescriber: Fulfill your professional responsibility by staying current with REMS-compliant education to ensure you are safely managing patients who require this level of pain management.
- If you are a pharmacist: Always provide the Medication Guide and use your expertise to counsel patients on the unique dangers and handling requirements of fentanyl patches.
By understanding its purpose, the REMS program empowers you to handle this powerful medication with the necessary caution and respect.
Summary Table:
| Key Aspect | Details |
|---|---|
| Governing Body | U.S. Food and Drug Administration (FDA) |
| Program Name | Opioid Analgesic REMS (Risk Evaluation and Mitigation Strategy) |
| Primary Goal | Ensure benefits outweigh risks of misuse, overdose, and accidental exposure |
| Key Requirements | Prescriber education, pharmacist counseling, patient Medication Guides |
| Key Risks Addressed | Extreme potency, accidental exposure, misuse, and addiction |
Navigating complex drug safety regulations requires a reliable manufacturing partner. As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharmaceutical distributors and brands, we understand the critical importance of compliance and safety. Our technical expertise in custom R&D and development ensures your products meet the highest standards. Let us help you bring safe, effective transdermal solutions to market. Contact our experts today to discuss your project needs.
Visual Guide
Related Products
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Mugwort Wormwood Pain Relief Patch for Neck Pain
People Also Ask
- How does menthol work in the Reliever Patch? Dual-Action Pain Relief Explained
- What are the important warnings for using menthol topical? Safety Tips for Effective Pain Relief
- How should a menthol patch be applied? Follow These Steps for Safe & Effective Pain Relief
- What are common side effects of menthol patch? Key Risks & Safety Tips
- How does menthol in the patch work to relieve pain? Discover the Science Behind Fast-Acting Relief















