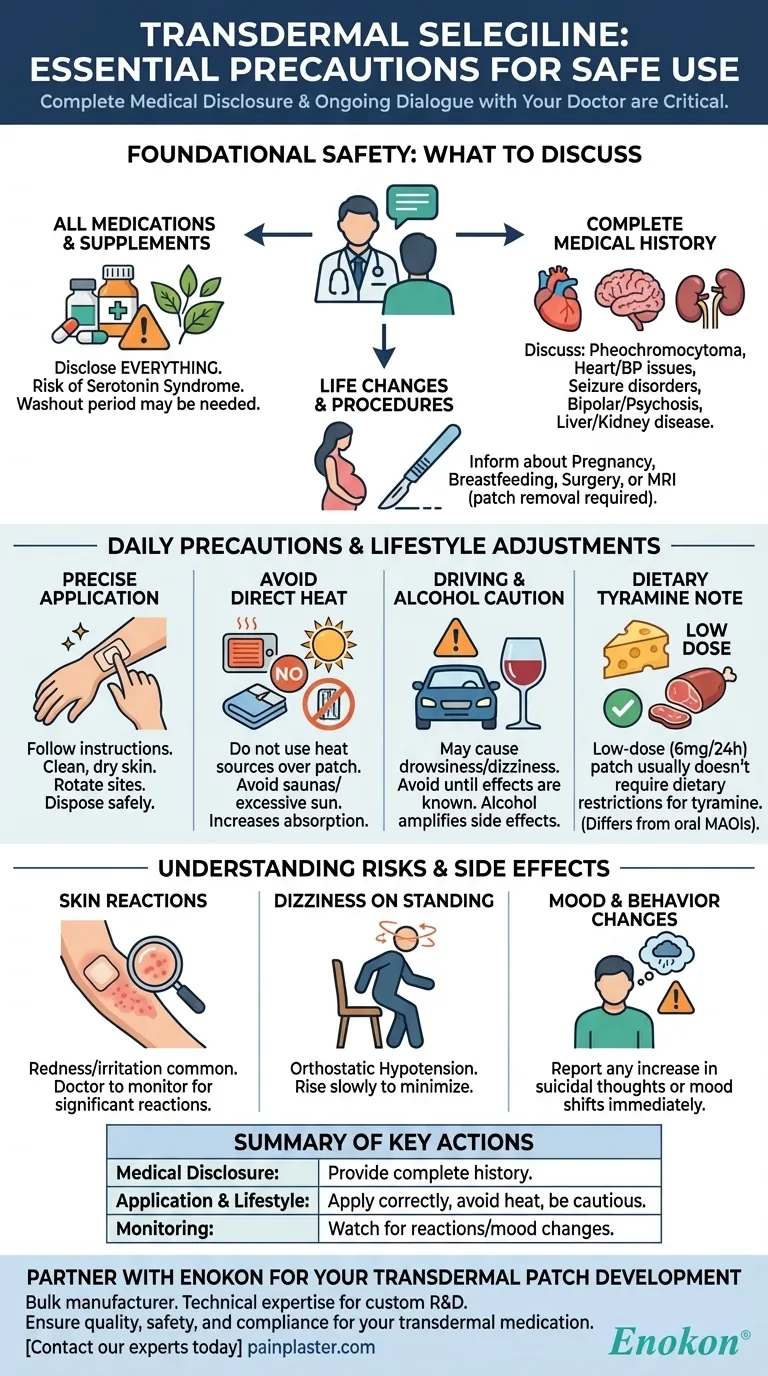
Before using transdermal selegiline, the most critical precautions involve providing your doctor with a complete inventory of your medical history and all current medications, including supplements. This transparency is essential to avoid severe drug interactions, such as serotonin syndrome or a hypertensive crisis, and to manage potential side effects related to blood pressure and mood.
Your safety while using the selegiline patch is not just about how you apply it; it hinges on a thorough and ongoing dialogue with your healthcare provider about your entire health profile to prevent potentially dangerous interactions.

Foundational Safety: What to Discuss With Your Doctor
Before your first prescription is written, a comprehensive medical review is the primary safety check. Selegiline interacts with numerous conditions and substances, making full disclosure non-negotiable.
Disclose All Medications and Supplements
You must inform your doctor about every medication you have taken recently or are currently taking. This includes prescription drugs, over-the-counter medicines, and herbal supplements.
Interactions with other antidepressants, stimulants, and certain pain relievers can lead to a life-threatening condition called serotonin syndrome. A "washout period" of 2 to 5 weeks is often required when switching from another antidepressant.
Review Your Complete Medical History
Certain pre-existing conditions require careful consideration before using selegiline. Be sure to discuss any history of:
- Pheochromocytoma (an adrenal gland tumor)
- Heart disease or high blood pressure
- Seizure disorders
- Bipolar disorder or a history of psychosis
- Liver or kidney disease
Inform Them of Planned Procedures or Life Changes
Let your medical team know if you are pregnant, planning to become pregnant, or breastfeeding.
You should also inform them of any upcoming surgeries or if you are scheduled for an MRI, as the patch may need to be removed beforehand.
Daily Precautions and Lifestyle Adjustments
Once you begin treatment, safety depends on proper daily use and awareness of how the medication affects your body.
Follow Application Instructions Precisely
Apply the patch to a clean, dry, and unbroken area of skin as directed. Wash your hands after application and use only one patch at a time unless specifically instructed otherwise.
Used patches still contain active medication and must be folded and disposed of safely, out of reach of children and pets.
Avoid Direct Heat Exposure
Do not use heating pads, electric blankets, or other direct heat sources over the patch. Excessive heat from saunas or prolonged sun exposure should also be avoided, as it can increase the rate of drug absorption to dangerous levels.
Be Cautious with Driving and Alcohol
Selegiline can cause drowsiness, dizziness, or fainting, particularly when you first start treatment. Avoid driving or operating heavy machinery until you know how the medication affects you.
Alcohol can amplify these side effects and should be avoided.
A Note on Dietary Tyramine
A significant advantage of the low-dose (6 mg/24 hours) transdermal patch is that dietary restrictions for tyramine-rich foods (like aged cheeses and cured meats) are generally not required. This is a key difference from older oral MAOIs.
Understanding the Risks and Side Effects
Being aware of potential adverse effects allows you to report them quickly and prevent complications.
Skin Reactions at the Application Site
Redness and irritation at the patch site are common. Your doctor should perform periodic skin examinations to monitor for any significant reactions or allergic responses.
Dizziness When Standing Up
The patch can cause a drop in blood pressure upon standing (orthostatic hypotension), leading to dizziness or fainting. To minimize this, rise slowly from a sitting or lying position.
Potential for Increased Suicidal Thoughts
Like many antidepressants, selegiline carries a warning about a potential increase in suicidal thoughts or behaviors, particularly in younger adults. Any changes in mood or behavior should be reported to your doctor immediately.
Making the Right Choice for Your Goal
Proactive communication and adherence to instructions are the cornerstones of using transdermal selegiline safely and effectively.
- If your primary focus is starting treatment safely: Your most critical action is providing your doctor with a complete list of every medication, supplement, and health condition you have.
- If your primary focus is safe daily use: Adhere strictly to application guidelines, avoid exposing the patch to direct heat, and be cautious about activities like driving until you understand its effects.
- If your primary focus is avoiding interactions: Always consult your doctor or pharmacist before starting any new medication, including over-the-counter products, while using the selegiline patch.
Ultimately, partnering closely with your healthcare provider is the single most important factor in ensuring your treatment is both safe and successful.
Summary Table:
| Key Precaution Category | Critical Actions to Take |
|---|---|
| Medical Disclosure | Provide a complete list of all medications, supplements, and medical conditions to your doctor. |
| Application & Lifestyle | Apply patch correctly, avoid direct heat, and be cautious with driving/alcohol. |
| Monitoring & Awareness | Watch for skin reactions, dizziness, and mood changes; report any concerns immediately. |
Partner with Enokon for Your Transdermal Patch Development
As a bulk manufacturer of reliable transdermal patches for healthcare and pharmaceutical distributors and brands, Enokon provides the technical expertise necessary for safe and effective custom R&D. If you are developing a transdermal medication like selegiline, our team can help ensure your product meets the highest standards of quality, safety, and patient compliance.
Contact our experts today to discuss your custom transdermal patch project and benefit from our specialized manufacturing capabilities.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Herbal Eye Protection Patch Eye Patch
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism















