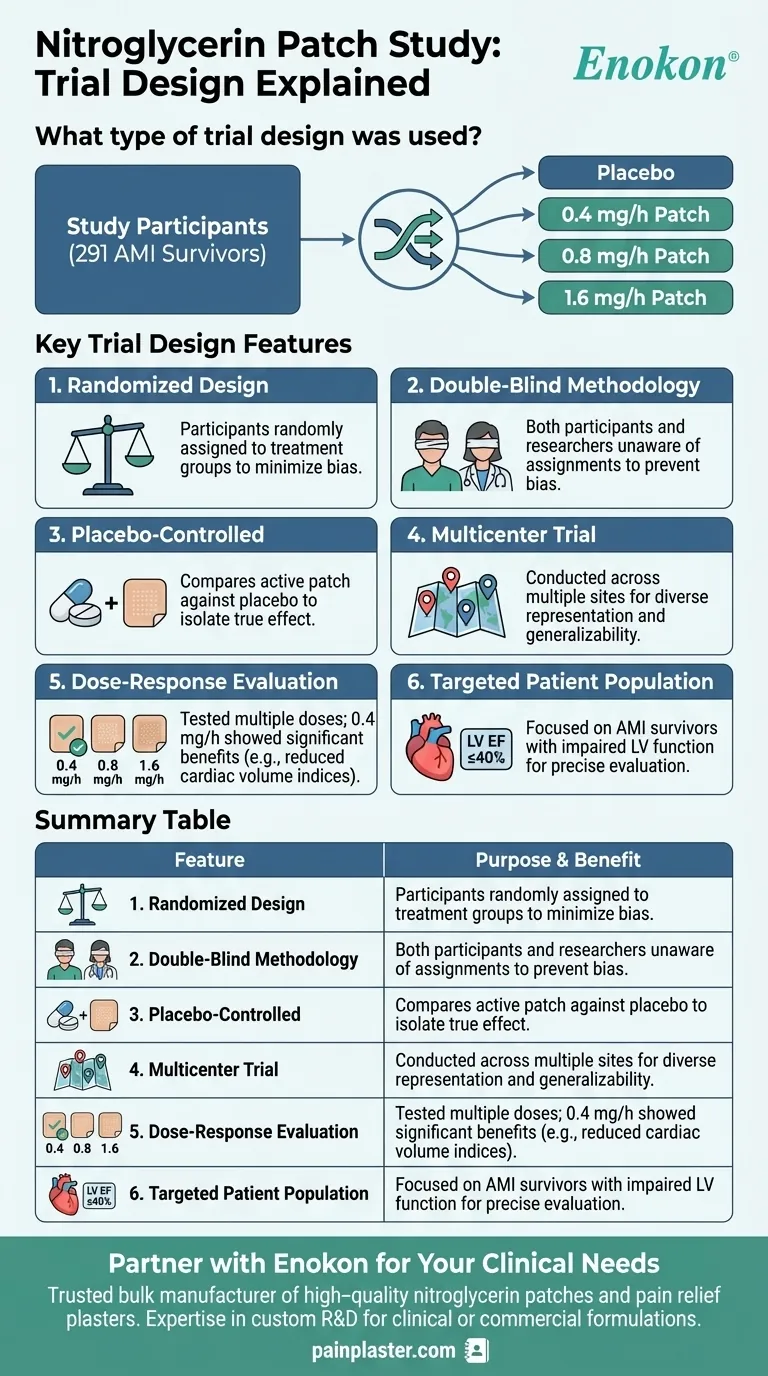
The study on the Nitroglycerin Patch employed a randomized, double-blind, placebo-controlled, multicenter trial design. It involved 291 acute myocardial infarction (AMI) survivors and compared placebo against three active nitroglycerin patch doses (0.4, 0.8, and 1.6 mg/h). The primary focus was to evaluate the efficacy of different doses in reducing cardiac volume indices, with the 0.4-mg/h dose showing statistically significant benefits in patients with baseline left ventricular ejection fraction ≤40%.
Key Points Explained:
-
Randomized Design
- Participants were randomly assigned to either the placebo group or one of the three active treatment groups (0.4, 0.8, or 1.6 mg/h).
- Randomization minimizes selection bias and ensures balanced distribution of confounding variables across groups.
-
Double-Blind Methodology
- Both participants and researchers were unaware of treatment assignments to prevent bias in outcome assessment or reporting.
- This design enhances the reliability of the results by eliminating placebo effects and observer bias.
-
Placebo-Controlled
- The inclusion of a placebo group allowed for direct comparison to isolate the true therapeutic effect of the Nitroglycerin Patch.
- Placebo-controlled trials are critical in evaluating whether observed effects are due to the intervention or other factors.
-
Multicenter Trial
- Conducted across multiple sites to ensure diverse participant representation and improve generalizability of findings.
- Multicenter studies also enhance recruitment efficiency and statistical power.
-
Dose-Response Evaluation
- The study tested three doses (0.4, 0.8, and 1.6 mg/h) to identify the optimal therapeutic dose.
- Only the 0.4-mg/h dose showed significant reductions in end-systolic and end-diastolic volume indices, particularly in patients with impaired baseline LV function.
-
Patient Population
- Focused on AMI survivors, a high-risk group for ventricular remodeling, to assess the patch’s potential in preventing adverse cardiac outcomes.
- Subgroup analysis revealed greater benefits in patients with LV ejection fraction ≤40%, highlighting targeted efficacy.
This rigorous trial design ensures robust conclusions about the Nitroglycerin Patch's efficacy, particularly for specific patient subgroups.
Summary Table:
| Trial Design Feature | Purpose & Benefit |
|---|---|
| Randomized | Ensures balanced group distribution and minimizes selection bias. |
| Double-Blind | Eliminates bias by keeping participants and researchers unaware of treatment groups. |
| Placebo-Controlled | Isolates the true therapeutic effect of the nitroglycerin patch. |
| Multicenter | Enhances participant diversity and improves generalizability of results. |
| Dose-Response Testing | Identifies optimal therapeutic dose (0.4 mg/h showed significant benefits). |
| Targeted Population | Focused on AMI survivors with LV dysfunction for precise efficacy evaluation. |
Need reliable transdermal patches for clinical trials or commercial use? Partner with Enokon, a trusted bulk manufacturer of high-quality nitroglycerin patches and pain relief plasters. Our expertise in custom R&D ensures formulations tailored to your clinical or commercial needs. Contact us today to discuss your project requirements!
Visual Guide
Related Products
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heating Pain Relief Patches for Menstrual Cramps
People Also Ask
- How does Pain Relief Patch deliver its active ingredients? | Targeted Relief Explained
- Can children use the pain relief patch? A Critical Safety Guide for Parents
- What are the key components of a pain relief patch? Unlock the Science of Targeted Pain Relief
- What are the benefits of Pain Relief Patch being licensed as a medicine? Guaranteed Efficacy & Safety
- Can the pain relief patch be used with other external analgesic products? A Critical Safety Guide

















