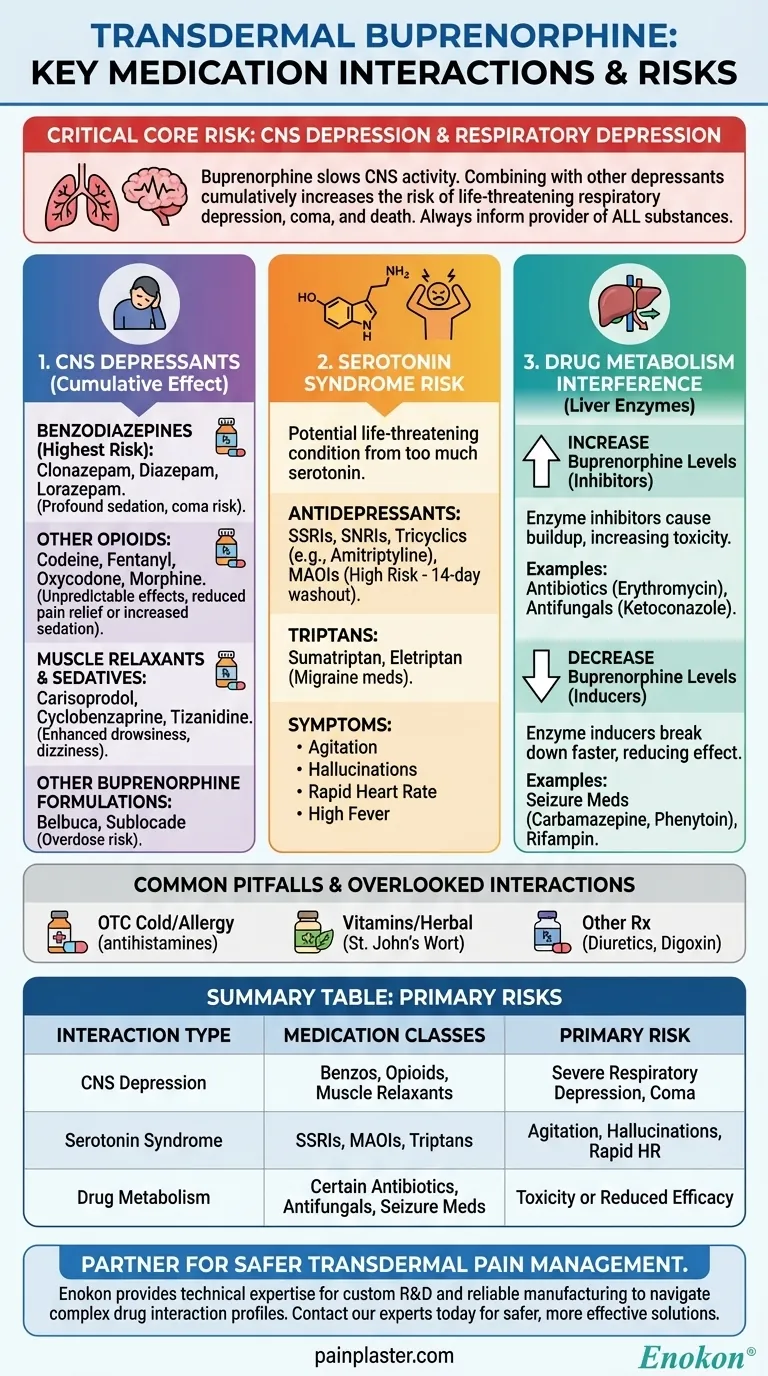
Critically, transdermal buprenorphine interacts with a wide range of medications, particularly those that also affect the central nervous system. The most dangerous combinations involve other central nervous system (CNS) depressants like benzodiazepines and other opioids, as well as certain antidepressants like MAO inhibitors, which can lead to life-threatening side effects. Always inform your healthcare provider of every medication, supplement, and substance you use.
The core danger of buprenorphine drug interactions is the risk of compounding its primary side effect: respiratory depression, or severely slowed breathing. Understanding which drug categories amplify this effect is the key to using this medication safely.
The Primary Risk: Central Nervous System (CNS) Depression
Buprenorphine works by depressing the central nervous system, which slows down brain activity, breathing, and heart rate. The most severe interactions occur with other substances that do the same thing, creating a dangerous cumulative effect.
Benzodiazepines
Combining buprenorphine with benzodiazepines is exceptionally risky. This combination significantly increases the risk of profound sedation, respiratory depression, coma, and death.
Common benzodiazepines include clonazepam, diazepam, and lorazepam.
Other Opioids
Using buprenorphine with other opioids can lead to unpredictable effects. It may increase sedation and respiratory depression or, because buprenorphine is a partial agonist, it could block the effects of other opioids and reduce pain relief.
Examples include codeine, fentanyl, oxycodone, and morphine.
Muscle Relaxants and Sedatives
Like the substances above, muscle relaxants and other sedating medicines add to the overall depressive load on the central nervous system. This enhances drowsiness, dizziness, and the risk of accidents.
This category includes drugs like carisoprodol, cyclobenzaprine, and tizanidine.
Other Buprenorphine Formulations
Using the transdermal patch with other medications containing buprenorphine, such as Belbuca or Sublocade, can easily lead to an overdose.
Serotonin Syndrome: A Different Kind of Risk
Some medications, when combined with buprenorphine, can cause a potentially life-threatening condition called serotonin syndrome. This occurs when there is too much serotonin, a natural chemical, in your body.
Antidepressants (SSRIs, SNRIs, Tricyclics)
Many common antidepressants work by increasing serotonin levels. This creates a risk when combined with buprenorphine, which can also affect serotonin pathways.
These include SSRIs, SNRIs, and tricyclic antidepressants like amitriptyline and nortriptyline.
Monoamine Oxidase Inhibitors (MAOIs)
This class of antidepressants presents a particularly high risk. Healthcare providers typically advise that you should not use buprenorphine if you have taken an MAOI within the past 14 days.
Triptans
Triptans, often prescribed for migraines, also affect serotonin levels and can contribute to the risk of serotonin syndrome when taken with buprenorphine. Examples include sumatriptan and eletriptan.
Interactions Affecting Drug Metabolism
Some medications can interfere with the liver enzymes responsible for breaking down buprenorphine. This can alter the concentration of the drug in your bloodstream, making it either too strong or too weak.
Medications that Increase Buprenorphine Levels
Certain drugs inhibit the enzymes that process buprenorphine, causing it to build up in your body. This increases the risk of side effects and toxicity.
Key examples include the antibiotic erythromycin and the antifungal ketoconazole.
Medications that Decrease Buprenorphine Levels
Other drugs induce these same enzymes, causing your body to break down buprenorphine too quickly. This can reduce its effectiveness and potentially lead to withdrawal symptoms.
This includes seizure medications like carbamazepine and phenytoin, as well as the antibiotic rifampin.
Common Pitfalls and Overlooked Interactions
Safe medication use requires total transparency. The most common pitfall is failing to disclose everything you take, as even seemingly harmless substances can cause significant interactions.
Over-the-Counter (OTC) Products
Many OTC cold and allergy medications contain sedating antihistamines or other ingredients that can increase the drowsiness caused by buprenorphine.
Vitamins and Herbal Supplements
Supplements are not inert. Products like St. John's Wort, for example, can interfere with drug metabolism and should be discussed with your doctor.
Other Prescription Medications
Several other classes of drugs can interact, including diuretics (water pills), anticholinergic medicines, and the heart medication digoxin. Always assume an interaction is possible.
Making the Right Choice for Your Safety
Proactive and honest communication with your healthcare team is the only way to manage these risks effectively.
- If your primary focus is starting treatment: Provide your doctor and pharmacist with a complete and detailed list of every prescription drug, over-the-counter product, vitamin, and herbal supplement you take before applying your first patch.
- If your primary focus is ongoing safety: Never start, stop, or change the dose of any medication or supplement without first clearing it with the provider who prescribes your buprenorphine.
- If your primary focus is recognizing problems: Immediately report any new or worsening symptoms—especially excessive sleepiness, confusion, dizziness, or slowed breathing—to your doctor or seek emergency care.
Your active participation is the most critical factor in ensuring your treatment is both safe and effective.
Summary Table:
| Interaction Type | Medication Classes | Primary Risk |
|---|---|---|
| CNS Depression | Benzodiazepines, Other Opioids, Muscle Relaxants | Severe respiratory depression, sedation, coma, death |
| Serotonin Syndrome | SSRIs/SNRIs, MAOIs, Triptans | Agitation, hallucinations, rapid heart rate, high body temperature |
| Drug Metabolism | Certain Antibiotics, Antifungals, Seizure Medications | Increased toxicity or reduced effectiveness of buprenorphine |
Ensure the safety and efficacy of your transdermal pain management products. As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon provides healthcare and pharma distributors and brands with the technical expertise for custom R&D and development. Let us help you navigate complex drug interaction profiles to create safer, more effective solutions for your patients. Contact our experts today to discuss your project requirements.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Pain Patch Relief Pain Reliever for Back
- Far Infrared Knee Pain Patch Heat Patches for Pain Relief
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- How do pain relief patches compare to other pain relief methods? Discover Targeted, Long-Lasting Relief
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief
- What are pain relief patches and how are they used? A Guide to Safe, Targeted Relief
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief














