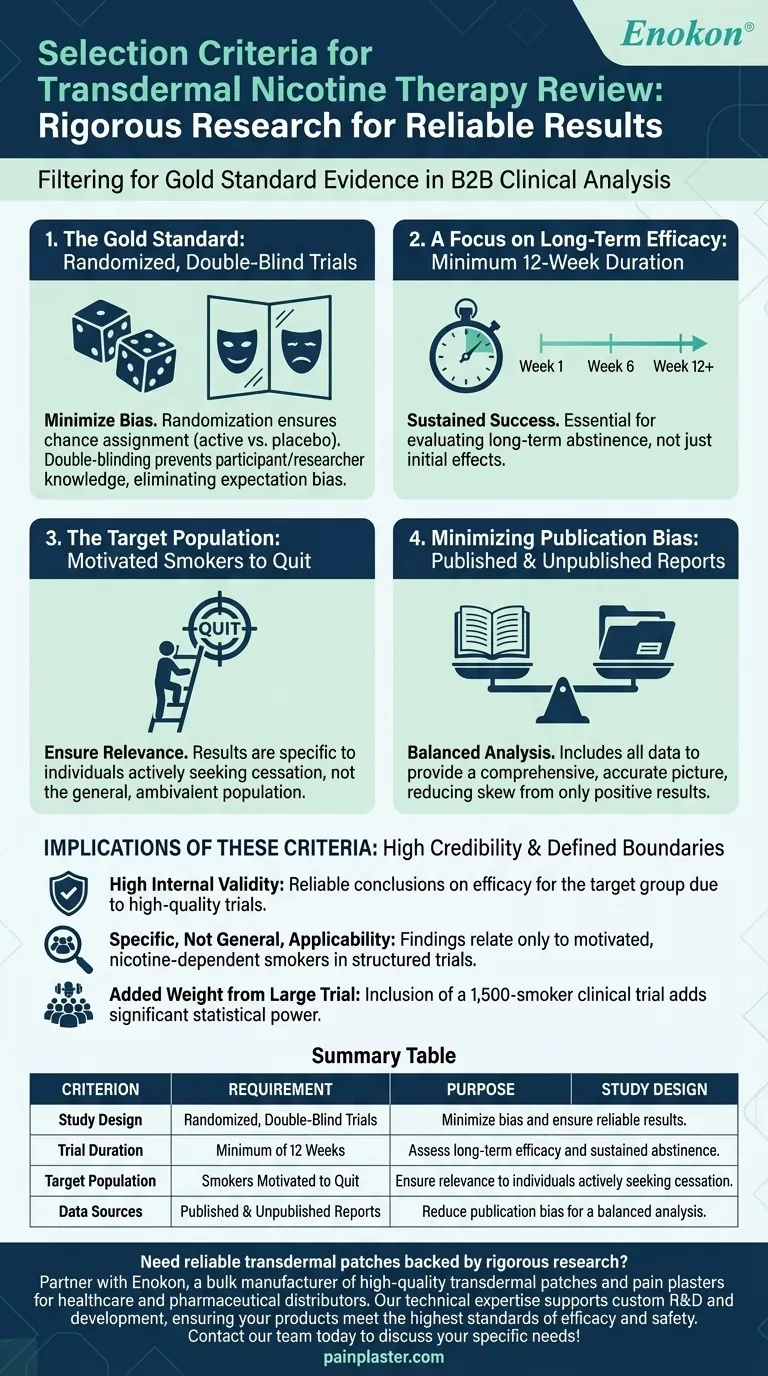
To be included in the review, studies on transdermal nicotine therapy had to be randomized, double-blind trials lasting for a minimum of 12 weeks. The review specifically targeted smokers who were motivated to quit and included both published and unpublished reports to provide a comprehensive analysis of the therapy's effectiveness.
The selection criteria were intentionally rigorous, focusing on the gold standard of clinical research to isolate the true effect of the therapy. This ensures the conclusions about efficacy are specifically relevant to nicotine-dependent smokers who are actively trying to quit.
Deconstructing the Selection Criteria
The strength of any research review lies in the quality of the studies it includes. The criteria used here were designed to gather the most reliable evidence available on transdermal nicotine therapy.
The Gold Standard: Randomized, Double-Blind Trials
The primary requirement was that studies be randomized, double-blind trials. This is the most robust type of clinical study design.
Randomization ensures that participants are assigned to either the nicotine patch or a placebo by chance, minimizing selection bias.
Double-blinding means that neither the participants nor the researchers know who is receiving the active treatment. This prevents expectations from influencing the results.
A Focus on Long-Term Efficacy
Studies were required to have a duration of at least 12 weeks. This criterion is critical for evaluating smoking cessation therapies.
Shorter trials might show an initial effect but fail to capture whether individuals can maintain abstinence over time. A 12-week minimum provides more meaningful data on sustained success.
The Target Population: Motivated Smokers
The review exclusively included trials conducted in smokers motivated to cease smoking.
This is a crucial distinction. The results and conclusions of this review are applicable to individuals who are actively seeking to quit, not the general population of smokers who may be ambivalent.
Minimizing Publication Bias
By including both published and unpublished reports, the review aimed to reduce publication bias.
Studies with positive or significant results are more likely to be published, which can skew the overall understanding of a therapy's effectiveness. Including unpublished data provides a more balanced and accurate picture.
The Implications of These Criteria
The strict selection process gives the review's findings a high degree of credibility, but it also defines the boundaries of its conclusions.
High Internal Validity
Because the review is built on high-quality trials, its conclusions about the efficacy of transdermal nicotine for the target group are very reliable. The methodology filters out noise and confounding factors.
Specific, Not General, Applicability
The findings cannot be generalized to all smokers. The proven effectiveness relates specifically to motivated, nicotine-dependent smokers participating in a structured trial. The results may differ in a broader, less motivated population.
Added Weight from a Large Trial
The specific inclusion of a clinical trial of 1500 smokers adds significant statistical power and real-world weight to the review's findings, reinforcing the conclusions drawn from the other included studies.
How to Interpret These Findings
Understanding the methodology allows you to apply the review's conclusions accurately.
- If your primary focus is clinical application: Recognize that this therapy's proven efficacy is strongly linked to a patient population that is already motivated to quit.
- If your primary focus is research: Note that the inclusion of unpublished data and the 12-week minimum duration set a high standard for future comparative effectiveness studies.
- If your primary focus is assessing credibility: The strict selection of randomized, double-blind trials confirms that the review's conclusions are based on high-quality, reliable evidence.
By understanding these methodological choices, you can confidently and correctly apply the review's conclusions.
Summary Table:
| Criterion | Requirement | Purpose |
|---|---|---|
| Study Design | Randomized, Double-Blind Trials | Minimize bias and ensure reliable results. |
| Trial Duration | Minimum of 12 Weeks | Assess long-term efficacy and sustained abstinence. |
| Target Population | Smokers Motivated to Quit | Ensure relevance to individuals actively seeking cessation. |
| Data Sources | Published & Unpublished Reports | Reduce publication bias for a balanced analysis. |
Need reliable transdermal patches backed by rigorous research? Partner with Enokon, a bulk manufacturer of high-quality transdermal patches and pain plasters for healthcare and pharmaceutical distributors. Our technical expertise supports custom R&D and development, ensuring your products meet the highest standards of efficacy and safety. Contact our team today to discuss your specific needs!
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
- Menthol Gel Pain Relief Patch
People Also Ask
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief















