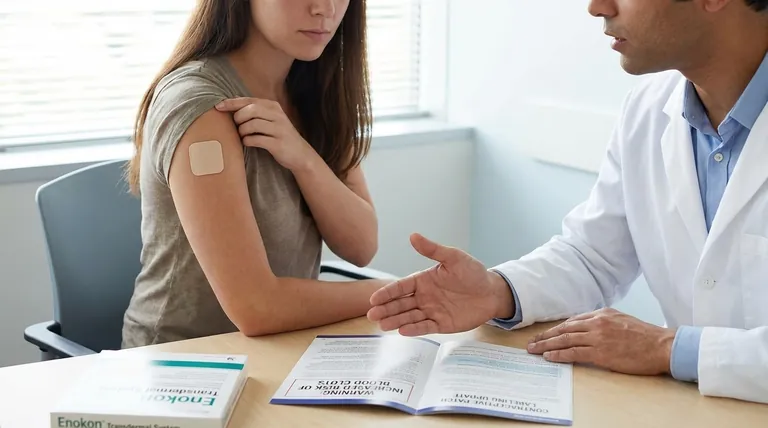
The critical update to the contraceptive patch labeling directly addresses the risk of blood clots. Regulators required a new warning stating that the patch exposes users to higher levels of estrogen than most combination oral contraceptive pills, which may increase the risk of developing a serious condition called venous thromboembolism (VTE).
The core issue is not simply the presence of estrogen, but the method of delivery. The patch releases a continuous and higher dose of estrogen into the bloodstream, which is directly linked to a potentially greater risk of blood clots compared to the fluctuating, lower doses from many daily pills.
Unpacking the Warning: Estrogen and VTE Risk
To understand the labeling change, we must look at how the contraceptive patch functions differently from traditional oral methods and why this difference matters for cardiovascular health.
What is Venous Thromboembolism?
Venous thromboembolism, or VTE, is a medical term for a blood clot that forms in a deep vein, most often in the leg (deep vein thrombosis or DVT).
The greatest danger occurs if a piece of this clot breaks off and travels to the lungs, causing a life-threatening blockage called a pulmonary embolism (PE).
The Role of Estrogen in Clotting
Estrogen, a key hormone in combined contraceptives, is known to affect the body's clotting system.
It can increase the concentration of certain proteins that help blood to clot, which slightly raises the baseline risk of VTE for anyone using a combined hormonal method.
The Patch vs. The Pill: A Key Difference
The crucial distinction lies in how the hormone is absorbed. An oral contraceptive pill is processed by the digestive system and liver before entering the bloodstream. This "first-pass metabolism" breaks down some of the estrogen.
The patch, however, delivers estrogen transdermally (through the skin) directly into the bloodstream. This bypasses the initial liver metabolism, resulting in a higher and more sustained level of estrogen exposure for the user. It is this elevated exposure that prompted the specific warning.
Understanding the Trade-offs and Context
The warning does not mean the patch is unsafe for everyone, but it does highlight a critical trade-off between convenience and a specific risk profile that users must consider.
"Increased Risk" is Relative
It is essential to contextualize this risk. The overall, or "absolute," risk of VTE for a young, healthy individual using hormonal contraception remains low.
The warning focuses on the relative risk—the patch may carry a higher risk when compared directly to some low-dose oral contraceptives.
Identifying Compounding Risk Factors
The risk associated with estrogen exposure is not uniform; it is significantly magnified by other personal health factors.
Key risk factors that compound the VTE risk from the patch include smoking, being over the age of 35, having a personal or family history of blood clots, obesity, and prolonged immobility.
Making an Informed Contraceptive Choice
Choosing the right contraceptive involves a careful balance of effectiveness, convenience, and your personal health profile against potential risks.
- If your primary focus is minimizing hormonal risk: Discuss this specific warning with your doctor and explore lower-dose oral contraceptives or non-hormonal methods like the IUD.
- If your primary focus is convenience: Acknowledge the patch's weekly application benefit but be fully transparent with your provider about your personal risk factors for VTE.
- If you have any pre-existing risk factors for blood clots: The contraceptive patch is likely not the appropriate method for you, and a thorough medical evaluation is critical before choosing any hormonal option.
Ultimately, a comprehensive discussion with your healthcare provider is the definitive step to aligning your contraceptive method with your individual health needs.
Summary Table:
| Key Aspect | Details |
|---|---|
| Warning Added | Higher risk of venous thromboembolism (VTE) |
| Primary Reason | Transdermal delivery leads to higher, sustained estrogen levels |
| Key Difference | Bypasses first-pass liver metabolism, unlike oral pills |
| Key Risk Factors | Smoking, age over 35, personal/family history of clots, obesity |
For Healthcare & Pharma Brands: Ensure Your Transdermal Products are Safe and Compliant
Navigating regulatory warnings and ensuring patient safety is complex. At Enokon, we are a bulk manufacturer of reliable transdermal patches and pain plasters. Our technical expertise in custom R&D and development helps healthcare and pharma distributors create products with precise, controlled drug delivery. Let us help you develop safe, effective transdermal solutions that meet the highest standards.
Contact our experts today to discuss your custom patch development needs.
Visual Guide
Related Products
- Prostate Pain Kidney Health Care Patch for Men
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Menthol Gel Pain Relief Patch
People Also Ask
- What should be done if a testosterone patch is missed or falls off? Follow these simple timing rules for safety and consistency.
- How often should testosterone patches be applied? Daily Dosage & Best Practices
- What should be done if a testosterone patch falls off? A Guide to Maintaining Hormone Stability
- What should patients tell their doctor before using testosterone patches? A Guide to Safe Treatment
- What should be done before undergoing an MRI while using testosterone patches? Remove it to prevent serious burns.















