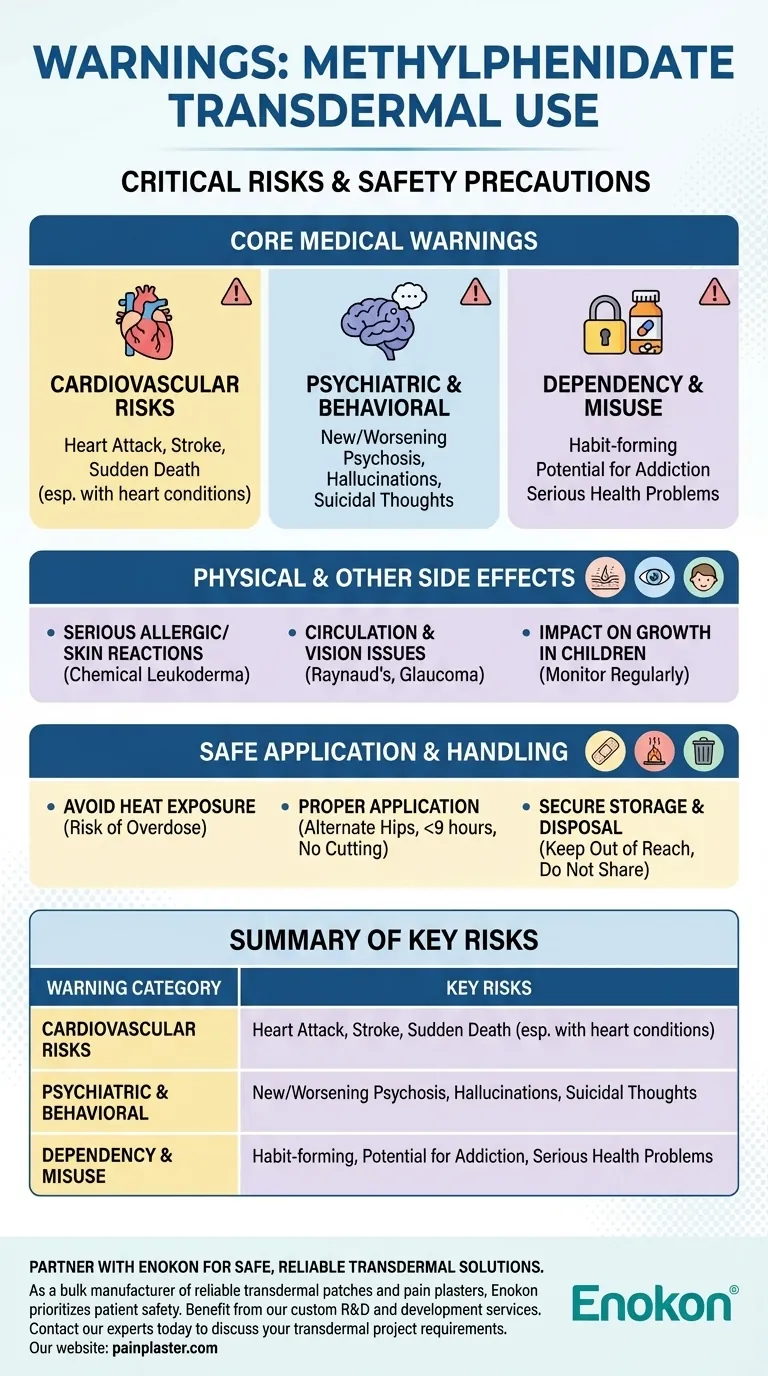
Before using transdermal methylphenidate, you must be aware of several critical warnings. The most serious risks include the potential for heart attack, stroke, or sudden death, especially in individuals with pre-existing heart conditions. Other significant warnings involve the risk of new or worsening psychiatric symptoms like psychosis, the habit-forming nature of the drug which can lead to addiction, and the need to closely monitor growth in children.
While transdermal methylphenidate can be an effective treatment for ADHD, its use carries significant cardiovascular, psychiatric, and dependency risks. These potential dangers underscore the absolute necessity for a thorough medical evaluation and strict adherence to prescribed guidelines.

Core Medical Warnings: What to Discuss With Your Doctor
A transparent conversation with your healthcare provider about your complete medical history is the first step in ensuring safety.
Cardiovascular Risks
The use of methylphenidate can increase blood pressure and heart rate. For individuals with high blood pressure, structural heart defects, or other serious heart problems, this can elevate the risk of heart attack, stroke, and even sudden death. It is critical to disclose any personal or family history of heart conditions before starting this medication.
Psychiatric and Behavioral Changes
This medication can cause or worsen psychiatric conditions. Patients may experience new or worsening psychosis, such as hearing voices or developing delusional beliefs. Other serious changes can include hallucinations, unusual shifts in behavior, or the emergence of suicidal thoughts.
Risk of Dependency and Misuse
Methylphenidate is a stimulant with a potential for misuse and addiction. It is classified as a habit-forming substance. Misuse of the drug can lead to serious health problems, including overdose, seizures, paranoia, or death. It should only be used exactly as prescribed and never shared with others.
Physical Side Effects and Reactions
Beyond the primary warnings, you must be aware of other serious physical reactions that require immediate medical attention.
Serious Allergic and Skin Reactions
An allergic reaction to methylphenidate can be severe. Additionally, the patch itself can cause serious skin reactions at the application site. A rare but serious side effect is chemical leukoderma, a permanent loss of skin color at the site where the patch was applied.
Circulation and Vision Issues
Some individuals may experience problems with blood flow, particularly in the fingers and toes, known as Raynaud's phenomenon. Other potential issues include changes in vision or the development or worsening of glaucoma.
Impact on Growth in Children
For children and adolescents using this medication, there is a risk of slowed growth and weight gain. A child's height and weight should be monitored regularly by their doctor throughout the treatment period.
Other Urgent Side Effects
Two other severe side effects require immediate medical help. These include priapism, a painful and prolonged erection lasting more than four hours, and the onset of seizures.
Understanding the Trade-offs: Safe Application is Non-Negotiable
How you handle and apply the patch directly impacts its safety and effectiveness. Deviating from the instructions can significantly increase risks.
The Danger of Heat Exposure
Do not expose the patch to direct heat sources like heating pads, electric blankets, or prolonged direct sunlight. Heat increases the rate at which the medication is absorbed into your body, which can lead to an accidental overdose.
Proper Application and Removal
Always apply the patch to the hip area, alternating hips each day to reduce skin irritation. The patch should never be worn for more than nine hours. If a patch becomes loose, do not use tape or other adhesives to reattach it. Crucially, never cut the patch, as this can alter the medication's dosage and delivery.
Secure Storage and Disposal
This medication must be stored securely and kept out of reach of children at all times. Because of its potential for misuse, it is vital that it is not shared with anyone for whom it was not prescribed.
Making an Informed Decision
Your specific health profile determines the level of risk and the necessary precautions for using transdermal methylphenidate.
- If you or your child have a history of heart conditions: A thorough cardiovascular evaluation is essential before starting this treatment.
- If there is a personal or family history of mental health issues: You must be vigilant for any new or worsening psychiatric symptoms and report them immediately.
- If you are concerned about safe daily use: Strict adherence to the rules of application, storage, and heat avoidance is critical to prevent accidental overdose.
Understanding these risks empowers you to work closely with your healthcare provider for the safest and most effective treatment possible.
Summary Table:
| Warning Category | Key Risks |
|---|---|
| Cardiovascular | Heart attack, stroke, sudden death (especially with pre-existing conditions) |
| Psychiatric | New/worsening psychosis, hallucinations, suicidal thoughts |
| Dependency | High potential for misuse, addiction, and serious health complications |
| Physical/Skin | Severe allergic reactions, chemical leukoderma (permanent skin color loss) |
| Application | Risk of overdose from heat exposure; improper use alters dosage |
Partner with Enokon for Safe, Reliable Transdermal Solutions
As a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharma distributors and brands, Enokon understands that patient safety is paramount. Our technical expertise ensures precise drug delivery and formulation stability, mitigating risks associated with transdermal medications.
Benefit from our custom R&D and development services to create patches that prioritize patient safety and efficacy. Contact our experts today to discuss your transdermal project requirements.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- How do pain relief patches provide targeted relief? Discover the Science Behind Effective Pain Management
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism













