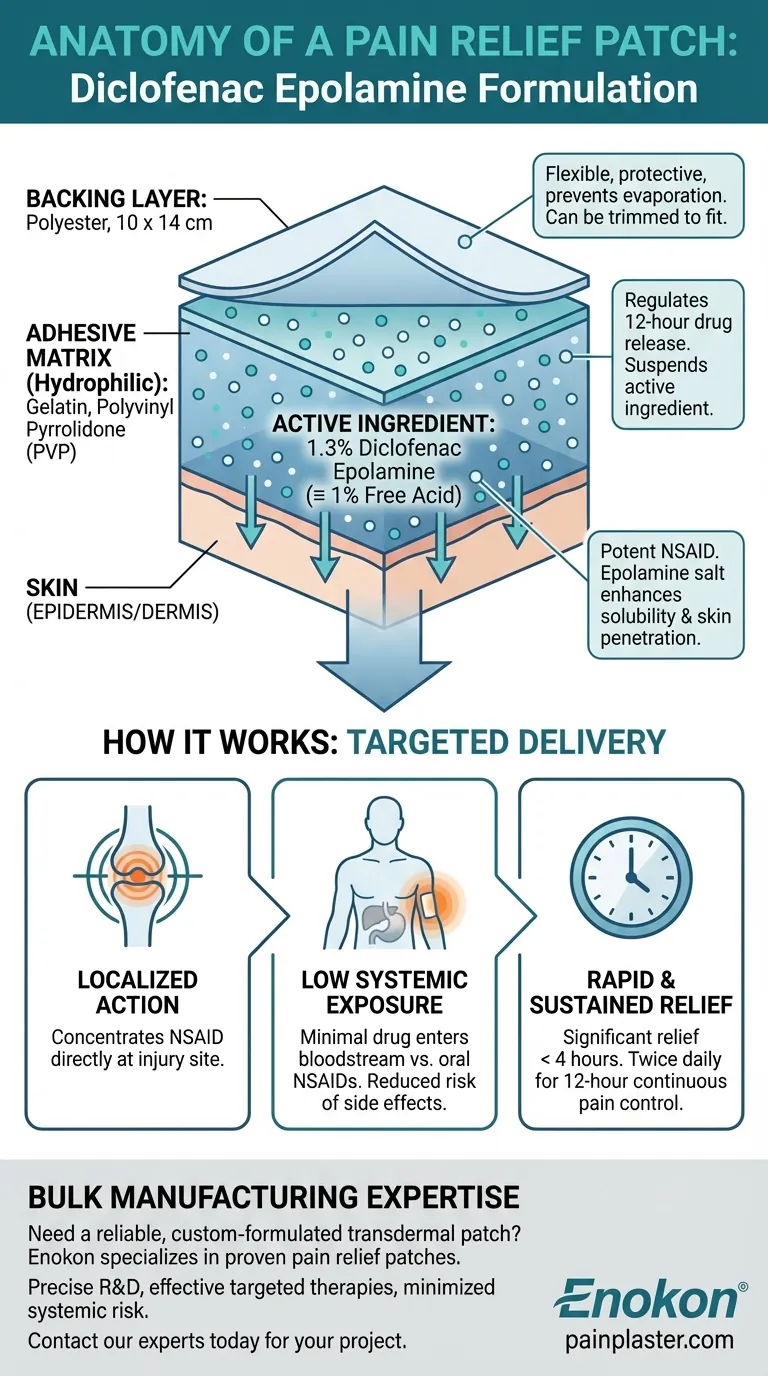
In the study you're referencing, the patch was composed of 1.3% diclofenac epolamine, which is equivalent to 1% diclofenac free acid. This active ingredient was embedded in a hydrophilic adhesive matrix containing gelatin, polyvinyl pyrrolidone, and other excipients, all applied to a 10 x 14 cm polyester backing.
The specific formulation of the diclofenac epolamine patch is engineered to deliver a potent NSAID directly to the site of pain, ensuring effective local relief while minimizing the systemic exposure and risks associated with oral medications.
Deconstructing the Patch Formulation
Understanding the components of the patch reveals how it achieves its therapeutic effect. Each element serves a distinct purpose, from the drug itself to the system that delivers it through the skin.
The Active Ingredient: Diclofenac Epolamine
The patch contains 1.3% diclofenac epolamine. Diclofenac is a well-established Non-Steroidal Anti-Inflammatory Drug (NSAID).
The epolamine salt form is specifically chosen for topical formulations. It enhances the solubility and skin penetration of the diclofenac, allowing it to reach the underlying painful tissues more effectively than the free acid form alone.
The Delivery System: Hydrophilic Adhesive
The active drug is suspended in a hydrophilic (water-loving) adhesive. This base is critical for the patch's function.
Key components like gelatin and polyvinyl pyrrolidone create a matrix that holds the drug and regulates its release over time. This system ensures a steady, 12-hour delivery of medication directly into the skin overlying the injury.
The Physical Structure: Backing and Dimensions
The entire formulation is applied to a flexible polyester backing. This layer protects the adhesive and drug from the environment and clothing, preventing the medication from evaporating or rubbing off.
Each patch measures 10 x 14 cm, providing coverage for a significant area. A key practical feature mentioned is the ability for users to trim the patch to fit a smaller area of pain, which prevents medication waste and improves conformity.
How This Formulation Delivers Targeted Relief
The patch's composition is not accidental; it is a purpose-built system designed to solve the primary problem of treating localized pain without impacting the entire body.
Localized Action, Minimized Systemic Risk
By delivering diclofenac directly through the skin, the patch concentrates the therapeutic effect at the injury site. This results in low systemic exposure, meaning very little of the drug enters the bloodstream.
This is a significant advantage over oral NSAIDs, which must be absorbed through the stomach, processed by the liver, and distributed throughout the body, increasing the risk of gastrointestinal and cardiovascular side effects.
Onset and Duration of Effect
The formulation provides both rapid and sustained relief. Studies show a significant reduction in pain within 4 hours of the first application.
With its twice-daily application schedule, each patch is designed to deliver its NSAID payload for 12 hours, providing consistent pain control throughout the day and night.
Key Considerations for Use
The design of the diclofenac epolamine patch directly translates into its clinical application and benefits.
Recommended Application
For acute injuries, the patch is applied directly to the skin over the painful area twice daily. This consistent application for up to 14 days ensures a steady state of pain and inflammation control at the target tissue.
Primary Advantages
The key features stemming from this formulation are its ability to provide targeted topical pain relief. It is fast-acting, offers sustained reduction in pain, and exists as an authorized generic, making it a more affordable option than the original brand while being identical in composition.
Making the Right Choice for Your Goal
- If your primary focus is rapid relief for an acute injury: The formulation is engineered for significant pain reduction within the first few hours of application.
- If your primary focus is sustained pain management with minimal side effects: The twice-daily patch provides 12 hours of targeted NSAID power, resulting in low systemic exposure compared to oral alternatives.
- If your primary focus is a practical and flexible treatment: The ability to trim the patch to the size of the painful area makes it an adaptable and efficient therapeutic option.
Ultimately, the specific composition of the diclofenac epolamine patch is designed to maximize local efficacy while minimizing systemic risk.
Summary Table:
| Component | Function | Key Detail |
|---|---|---|
| Active Ingredient | Provides NSAID pain relief | 1.3% diclofenac epolamine (equivalent to 1% free acid) |
| Adhesive Matrix | Regulates drug release over 12 hours | Hydrophilic base with gelatin & polyvinyl pyrrolidone |
| Backing & Size | Protects drug and allows customization | 10x14 cm polyester; can be trimmed to fit |
| Key Benefit | Localized action with minimal systemic exposure | Significant pain reduction within 4 hours |
Need a reliable, custom-formulated transdermal patch for your healthcare brand or distribution network? At Enokon, we specialize in bulk manufacturing of proven pain relief patches, like the diclofenac epolamine formulation discussed. Our technical expertise ensures precise R&D and development for your specific requirements, delivering effective, targeted therapies with minimized systemic risk.
Contact our experts today to discuss your custom transdermal patch project and benefit from our manufacturing excellence.
Visual Guide
Related Products
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Pain Patch Relief Pain Reliever for Back
- Far Infrared Knee Pain Patch Heat Patches for Pain Relief
- Herbal Eye Protection Patch Eye Patch
People Also Ask
- What types of coughs can the far infrared cough relief patch address? Soothe Dry, Wet, and Persistent Coughs
- What makes the cough relief patch a convenient option for managing coughs? A Mess-Free, On-the-Go Solution
- How does capsaicin work in the Reliever Patch? A Drug-Free Solution for Targeted Pain Relief
- What are the key benefits of using the cough relief patch? Soothe Your Cough with Targeted, Non-Oral Relief
- How should missed doses of the Reliever Patch be handled? Safe Usage Guidelines















