The historical significance of the nicotine patch is twofold: it provided the first FDA-approved, transdermal method for smoking cessation, and more importantly, it became the first "blockbuster" medication of its kind. This success proved the immense medical and commercial viability of delivering drugs through the skin, fundamentally altering the course of pharmaceutical development.
While famous for helping people quit smoking, the nicotine patch's greatest legacy was legitimizing transdermal drug delivery as a mainstream medical platform, paving the way for dozens of other skin-based medications.

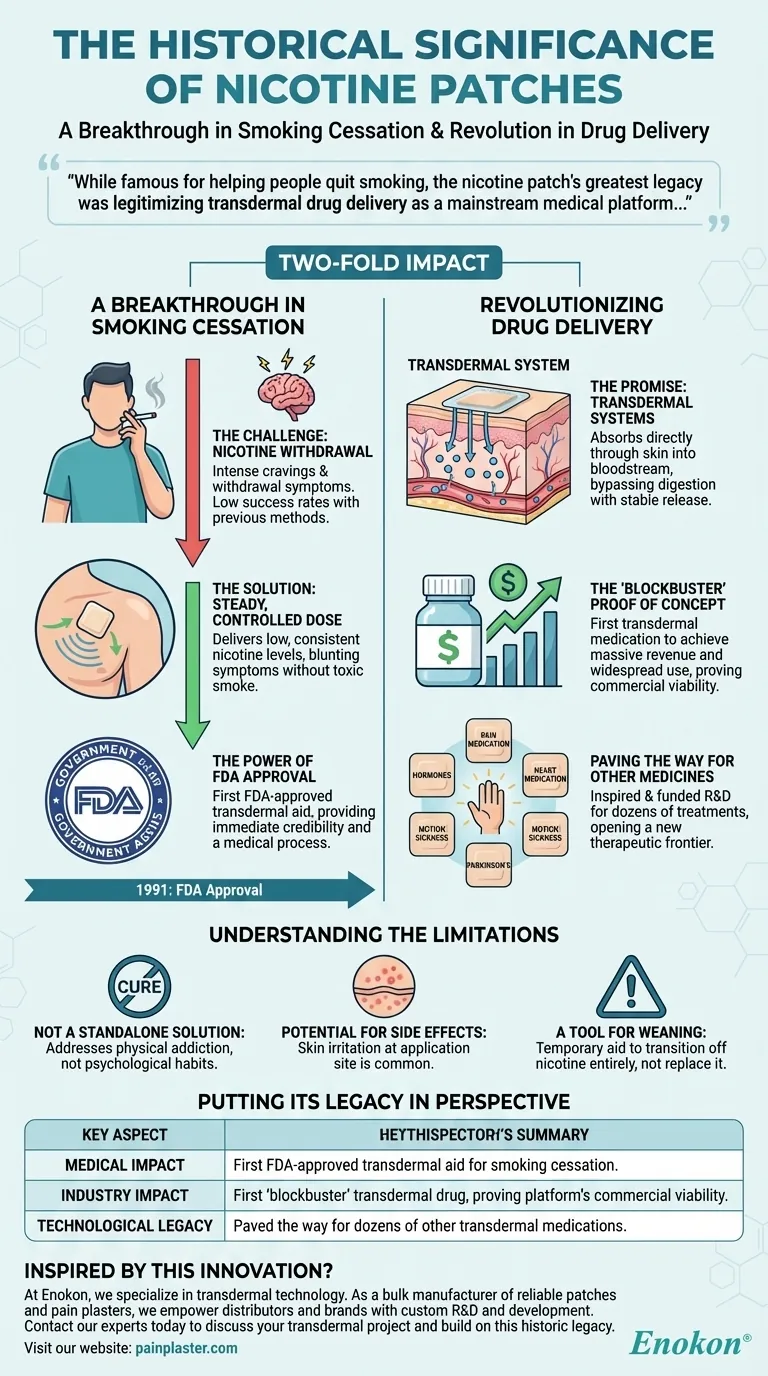
A Breakthrough in Smoking Cessation
Before the nicotine patch, options for quitting smoking were limited and often had low success rates. The patch represented a significant leap forward by directly addressing the physical component of addiction in a novel way.
The Challenge of Nicotine Withdrawal
The primary barrier to quitting is managing the intense cravings and withdrawal symptoms caused by nicotine addiction. Previous methods often provided inconsistent relief, leading to high rates of relapse.
Delivering a Steady, Controlled Dose
The patch's key innovation was its ability to deliver a low, steady dose of nicotine through the skin over many hours. This consistent supply helps to blunt withdrawal symptoms and reduce cravings without the toxic tar and carbon monoxide of cigarette smoke.
The Power of FDA Approval
As the first FDA-approved transdermal aid for smoking cessation, the nicotine patch gained immediate credibility with both doctors and the public. It transformed quitting from a matter of pure willpower into a manageable medical process with a proven tool.
Revolutionizing Drug Delivery
The patch’s impact extends far beyond public health. Its unprecedented success sent shockwaves through the pharmaceutical industry and established a new frontier for treatment.
The Promise of Transdermal Systems
Transdermal drug delivery involves absorbing medication directly through the skin into the bloodstream. This method can bypass the digestive system, avoid injections, and provide a very stable release of a drug over time.
The 'Blockbuster' Proof of Concept
The nicotine patch was the first transdermal medication to achieve blockbuster status, meaning it generated massive revenue and was used by millions. This success demonstrated that delivering medicine through the skin was not just a theoretical concept but a highly effective and profitable reality.
Paving the Way for Other Medicines
The success of the nicotine patch directly inspired and funded research and development into other transdermal treatments. Today, patches are used to deliver hormones, pain medication, heart medication, and treatments for conditions like motion sickness and Parkinson's disease.
Understanding the Limitations
Despite its historic impact, it's crucial to view the nicotine patch in its proper context. It was a revolutionary tool, not a cure-all for a complex addiction.
Not a Standalone Solution
The patch is designed to manage the physical addiction to nicotine. It does not address the powerful psychological or behavioral habits associated with smoking, which require separate strategies to overcome.
Potential for Side Effects
Like any medical treatment, the patch has potential side effects. The most common is skin irritation at the application site, a known trade-off for transdermal systems.
A Tool for Weaning, Not Replacing
The ultimate goal of Nicotine Replacement Therapy (NRT) is to wean the user off nicotine entirely. The patch is a temporary aid designed to make that transition process smoother and more successful.
Putting Its Legacy in Perspective
To understand the patch's significance, consider its impact from two key viewpoints.
- If your primary focus is public health: The nicotine patch was a landmark achievement in harm reduction, offering the first widely adopted medical tool to effectively combat tobacco addiction.
- If your primary focus is pharmacology: The patch is the pivotal product that validated the entire field of transdermal drug delivery, opening a new frontier for treatment that continues to expand today.
Ultimately, the nicotine patch didn't just change how people quit smoking; it fundamentally changed how medicine itself could be delivered.
Summary Table:
| Key Aspect | Historical Significance |
|---|---|
| Medical Impact | First FDA-approved transdermal aid for smoking cessation. |
| Industry Impact | First 'blockbuster' transdermal drug, proving the platform's commercial viability. |
| Technological Legacy | Paved the way for dozens of other transdermal medications for pain, hormones, and more. |
Inspired by the innovation of the nicotine patch?
At Enokon, we specialize in the very technology this breakthrough pioneered. As a bulk manufacturer of reliable transdermal patches and pain plasters, we empower healthcare and pharmaceutical distributors and brands to bring their own innovative treatments to market.
Benefit from our technical expertise for custom R&D and development. Whether you're creating a new smoking cessation aid, a hormone therapy, or a pain management solution, we provide the reliable manufacturing and development support you need to succeed.
Contact our experts today to discuss your transdermal project and discover how we can help you build on this historic legacy.
Visual Guide
Related Products
- Menthol Gel Pain Relief Patch
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- What are the important warnings for using menthol topical? Safety Tips for Effective Pain Relief
- What is the primary use of a menthol patch? Targeted Relief for Muscle & Joint Pain
- What are common side effects of menthol patch? Key Risks & Safety Tips
- How does menthol function as a topical analgesic? The Science Behind Cooling Pain Relief
- How does menthol in the patch work to relieve pain? Discover the Science Behind Fast-Acting Relief
















