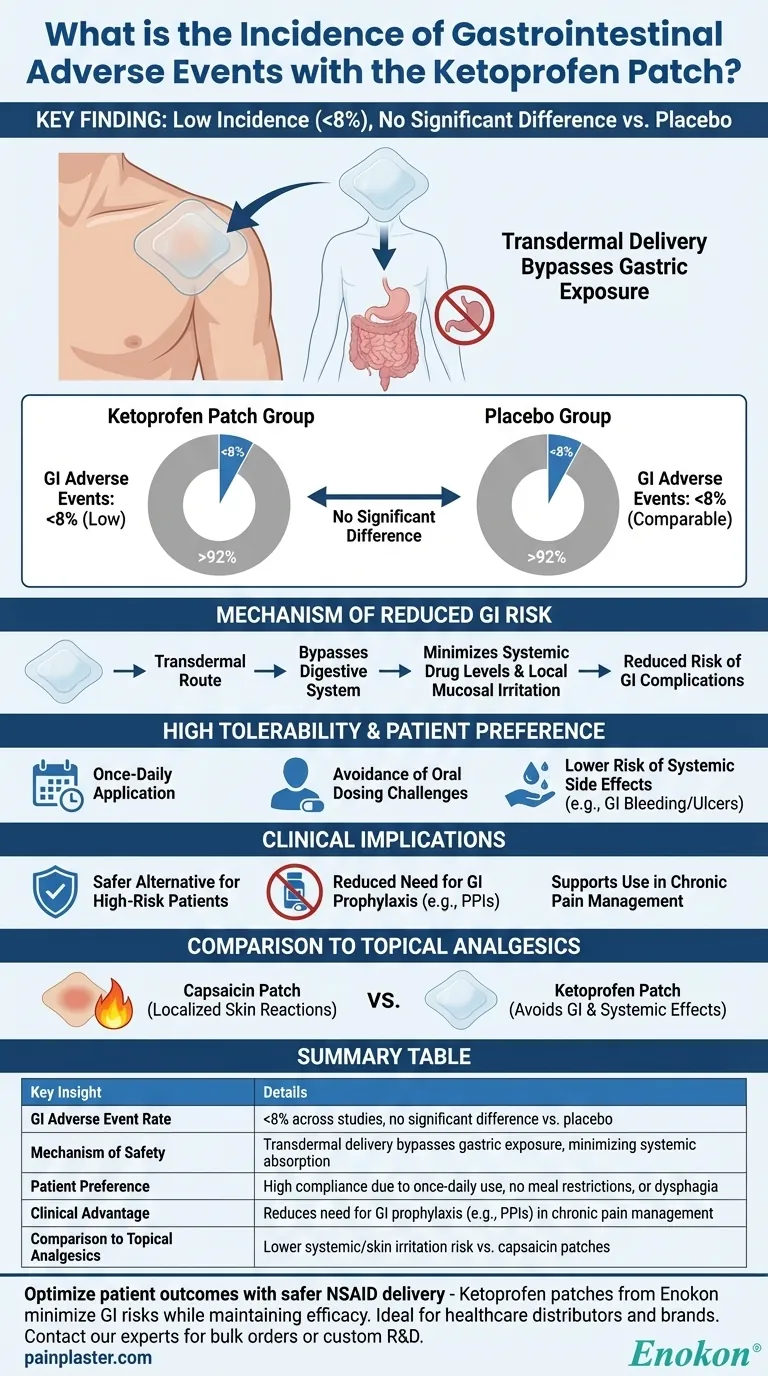
The incidence of gastrointestinal adverse events with ketoprofen patches was consistently reported as low (less than 8% of patients) across multiple studies, with no significant difference observed between the ketoprofen patch and placebo groups. This suggests that the transdermal delivery system effectively minimizes systemic exposure, reducing the risk of GI irritation commonly associated with oral NSAIDs. The patch format also demonstrated excellent tolerability, patient preference, and compliance due to its once-daily application and localized effects.

Key Points Explained:
-
Low Incidence of Gastrointestinal Adverse Events (<8%)
- Multiple studies consistently reported gastrointestinal adverse events occurring in less than 8% of patients using ketoprofen patches.
- This low rate is comparable to placebo, indicating minimal systemic absorption and reduced GI irritation risk compared to oral NSAIDs.
-
No Significant Difference vs. Placebo
- The similarity in GI adverse event rates between ketoprofen patches and placebo suggests the transdermal route avoids first-pass metabolism and direct gastric exposure.
- This is a critical advantage for patients with a history of NSAID-induced GI complications.
-
Mechanism of Reduced GI Risk
- Transdermal delivery bypasses the digestive system, minimizing systemic drug levels and local mucosal irritation.
- The patch’s localized action (e.g., for musculoskeletal pain) further limits unnecessary drug distribution.
-
High Tolerability and Patient Preference
- Studies highlighted excellent compliance and patient preference for the patch format due to:
- Once-daily application convenience.
- Avoidance of oral dosing challenges (e.g., dysphagia, timing with meals).
- Lower risk of systemic side effects like GI bleeding or ulcers.
- Studies highlighted excellent compliance and patient preference for the patch format due to:
-
Clinical Implications
- Ketoprofen patches offer a safer alternative for patients requiring NSAID therapy but prone to GI adverse events.
- The low incidence supports use in chronic pain management without routine GI prophylaxis (e.g., PPIs), reducing polypharmacy.
-
Comparison to Other Topical Analgesics
- Unlike capsaicin patches (which cause localized skin reactions), ketoprofen patches primarily avoid GI and systemic effects.
- This makes them suitable for patients sensitive to topical irritants but needing NSAID efficacy.
The data underscores how ketoprofen patches combine efficacy with a favorable safety profile, addressing a key limitation of traditional NSAID therapies. For healthcare purchasers, this translates to reduced complications and costs associated with GI adverse event management. Could this transdermal approach redefine long-term NSAID use in high-risk populations?
Summary Table:
| Key Insight | Details |
|---|---|
| GI Adverse Event Rate | <8% across studies, no significant difference vs. placebo. |
| Mechanism of Safety | Transdermal delivery bypasses gastric exposure, minimizing systemic absorption. |
| Patient Preference | High compliance due to once-daily use, no meal restrictions, or dysphagia. |
| Clinical Advantage | Reduces need for GI prophylaxis (e.g., PPIs) in chronic pain management. |
| Comparison to Topical Analgesics | Lower systemic/skin irritation risk vs. capsaicin patches. |
Optimize patient outcomes with safer NSAID delivery — Ketoprofen patches from Enokon minimize GI risks while maintaining efficacy. Ideal for healthcare distributors and brands seeking compliant, cost-effective pain management solutions. Contact our experts to discuss bulk orders or custom R&D for your transdermal product line.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Prostate Pain Kidney Health Care Patch for Men
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- What are pain relief patches and how are they used? A Guide to Safe, Targeted Relief
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief
- How do pain relief patches compare to other pain relief methods? Discover Targeted, Long-Lasting Relief
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief
















