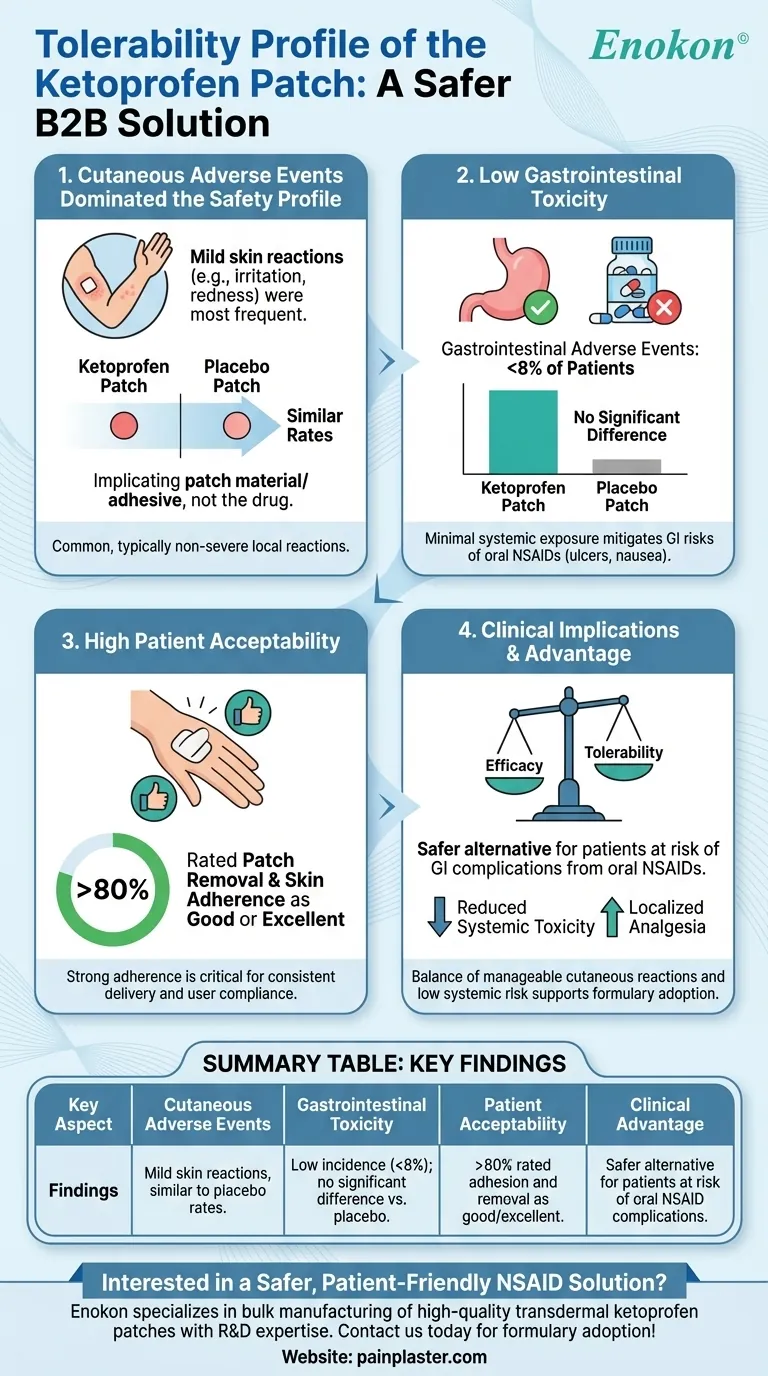
The ketoprofen patch demonstrated a favorable tolerability profile in clinical evaluations, with adverse events being primarily mild cutaneous reactions that occurred at comparable rates between the active treatment and placebo groups. This suggests that skin-related side effects were attributable to the patch itself rather than the ketoprofen patches. Gastrointestinal adverse events, a common concern with oral NSAIDs like ketoprofen, were notably low (<8% of patients) and similar between groups, highlighting a key advantage of transdermal delivery. Patient acceptability was high, with over 80% rating patch removal and skin adherence as good or excellent, supporting its practical use in pain management.
Key Points Explained:
-
Cutaneous Adverse Events Dominated the Safety Profile
- The most frequent side effects were skin-related (e.g., mild irritation, redness).
- Incidence rates were similar between ketoprofen and placebo patches, implicating the patch material or adhesive as the likely cause rather than the drug itself.
- This pattern aligns with transdermal systems, where local skin reactions are common but typically non-severe.
-
Low Gastrointestinal Toxicity
- Oral NSAIDs often cause GI disturbances (e.g., ulcers, nausea), but the patch showed minimal systemic exposure to ketoprofen.
- GI adverse events occurred in <8% of patients, with no significant difference between active and placebo groups.
- This underscores the patch’s potential to mitigate systemic side effects while delivering localized analgesia.
-
High Patient Acceptability
- Over 80% of users reported positive experiences with patch adhesion and ease of removal.
- Strong adherence properties are critical for consistent drug delivery and user compliance, especially in chronic pain management.
-
Clinical Implications
- The tolerability profile supports the patch as a safer alternative for patients at risk of GI complications from oral NSAIDs.
- Cutaneous reactions, while common, were manageable and did not outweigh the benefits of reduced systemic toxicity.
By minimizing systemic exposure and focusing on localized delivery, the ketoprofen patch addresses a key unmet need in NSAID therapy—balancing efficacy with tolerability. For purchasers, this translates to a product with lower risk of severe adverse events and higher patient satisfaction, factors that can streamline formulary adoption.
Summary Table:
| Key Aspect | Findings |
|---|---|
| Cutaneous Adverse Events | Mild skin reactions (e.g., irritation, redness); similar to placebo rates. |
| Gastrointestinal Toxicity | Low incidence (<8%); no significant difference vs. placebo. |
| Patient Acceptability | >80% rated adhesion and removal as good/excellent. |
| Clinical Advantage | Safer alternative for patients at risk of oral NSAID complications. |
Interested in a safer, patient-friendly NSAID solution? Enokon specializes in bulk manufacturing of high-quality transdermal ketoprofen patches, backed by technical expertise for custom R&D. Contact us today to discuss formulary adoption or tailored pain management products!
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Icy Hot Menthol Medicine Pain Relief Patch
- Prostate Pain Kidney Health Care Patch for Men
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Far Infrared Heat Pain Relief Patches Transdermal Patches
People Also Ask
- How do pain relief patches provide targeted relief? Discover the Science Behind Effective Pain Management
- How do pain relief patches compare to other pain relief methods? Discover Targeted, Long-Lasting Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief