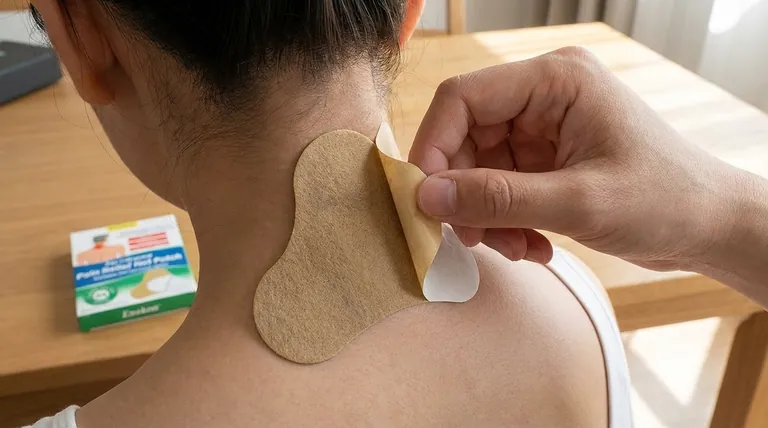
The topical ketoprofen patches demonstrated a favorable tolerability profile in clinical evaluations. Adverse events were primarily cutaneous (skin-related) and occurred at similar rates in both the ketoprofen and placebo groups, indicating these effects were likely due to the patch material rather than the active drug. Gastrointestinal adverse events were infrequent (<8% of patients) and comparable between treatment and placebo groups. Patient acceptability was high, with over 80% rating patch removal and skin adherence as good or excellent. The evidence suggests this formulation is a safe, effective option for managing localized painful inflammation with minimal systemic side effects.
Key Points Explained:
-
Cutaneous Adverse Events Dominance
- The most common adverse effects were skin-related (e.g., irritation, redness).
- Occurrence rates were nearly identical between ketoprofen and placebo patches, strongly implicating the patch material itself as the cause rather than the drug.
-
Low Gastrointestinal Tolerability Concerns
- Systemic absorption appears minimal, as GI events (typically associated with oral NSAIDs like ketoprofen) were rare (<8% incidence).
- No significant difference between active and placebo groups reinforces the localized action of the transdermal delivery system.
-
High Patient Acceptability
-
80% of users reported positive experiences with patch adhesion and removal.
- This practical tolerance complements the safety data, suggesting high adherence potential in real-world use.
-
-
Safety Advantages Over Systemic NSAIDs
- Unlike oral ketoprofen, the patch avoids first-pass metabolism, reducing risks of ulcers or renal complications.
- The cutaneous AE profile (mild and transient) is preferable to systemic NSAID toxicity.
-
Placebo-Controlled Evidence
- Consistent findings across multiple studies strengthen conclusions about patch-specific (not drug-specific) tolerability challenges.
- Researchers could confidently attribute non-cutaneous AEs to other factors given the placebo group data.
This profile positions ketoprofen patches as a compelling alternative for patients needing sustained anti-inflammatory action without compromising systemic safety. For purchasers, these tolerability metrics support cost-benefit analyses favoring transdermal NSAIDs over oral forms in eligible populations.
Summary Table:
| Key Aspect | Findings |
|---|---|
| Cutaneous Adverse Events | Mostly skin-related (e.g., irritation), similar rates in ketoprofen & placebo groups. |
| Gastrointestinal Events | Rare (<8% incidence), no significant difference vs. placebo. |
| Patient Acceptability | >80% rated patch adhesion & removal as good/excellent. |
| Safety vs. Oral NSAIDs | Avoids first-pass metabolism, reducing ulcer/renal risks. |
| Evidence Strength | Consistent placebo-controlled data supports localized tolerability. |
Looking for reliable transdermal pain relief solutions?
Enokon specializes in bulk manufacturing of high-quality ketoprofen patches and custom transdermal formulations for healthcare distributors and brands. Benefit from our technical expertise in R&D to develop tailored, patient-friendly pain management products.
Contact us today to discuss your project needs!
Visual Guide

Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Prostate Pain Kidney Health Care Patch for Men
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- How do pain relief patches compare to other pain relief methods? Discover Targeted, Long-Lasting Relief
- How do pain relief patches provide targeted relief? Discover the Science Behind Effective Pain Management
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief















