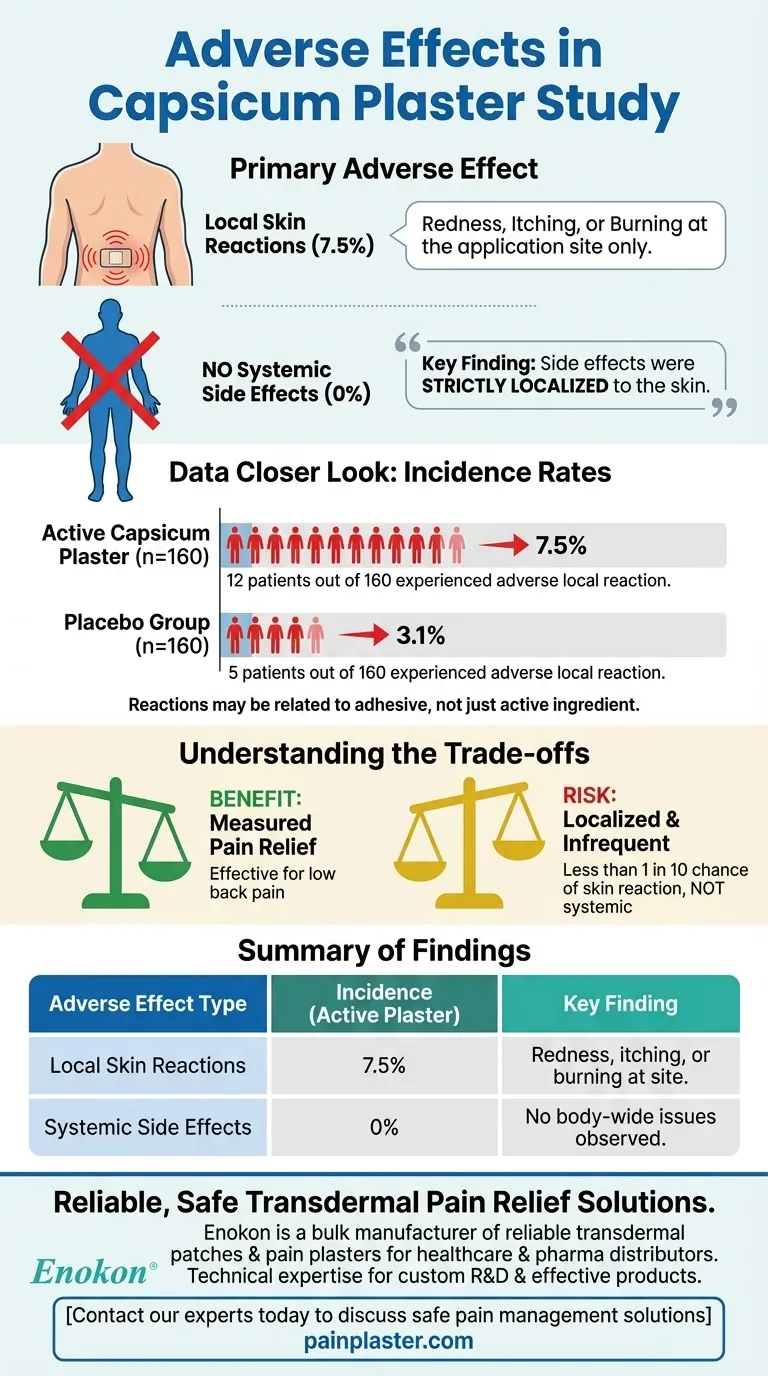
The primary adverse effects observed in the capsicum plaster study were adverse local drug reactions, which occurred in 7.5% of patients using the active plaster. Importantly, the study found no evidence of any systemic, or body-wide, side effects.
The key finding is that the side effects associated with capsicum plaster in this study were strictly localized to the skin where the plaster was applied and were not widespread throughout the body.

Primary Findings: A Closer Look at the Data
The study was designed to measure not only the effectiveness of the capsicum plaster for pain relief but also its safety and tolerance compared to a placebo. The results on adverse effects were clear and specific.
Incidence Rate
A total of 12 patients out of 160 (or 7.5%) who used the active capsicum plaster experienced an adverse local reaction. This provides a specific measure of how frequently these side effects occurred within the trial.
Comparison with Placebo
For context, 5 patients out of 160 (or 3.1%) in the placebo group also reported adverse local reactions. This indicates that some of the reactions may be related to the plaster or adhesive itself, rather than the active capsicum ingredient.
The Nature of the Reactions
The reactions were described exclusively as "local." This means the effects were confined to the area of skin directly covered by the plaster. Common examples of local reactions include redness, itching, or a burning sensation.
Absence of Systemic Effects
A critical finding for safety was the complete absence of systemic side effects. This means the active ingredient did not cause issues throughout the body, such as digestive problems, dizziness, or other internal complications.
Understanding the Trade-offs
When evaluating any treatment, it is essential to weigh the potential benefits against the risks. This study provides a clear framework for understanding that balance with capsicum plasters.
The Benefit: Measured Pain Relief
The study's main purpose was to assess efficacy. It used several measures, including pain scores and mobility indexes, to confirm that the capsicum plaster provided tangible pain relief for low back pain.
The Risk: Localized and Infrequent
The primary risk identified was a less than 1 in 10 chance of experiencing a local skin reaction. This risk is not systemic, meaning it does not affect internal organs or the body as a whole.
What the Study Doesn't Specify
The provided information does not detail the specific types of local reactions (e.g., rash, itching, burning) or their severity. It only quantifies their occurrence.
Making the Right Choice for Your Goal
Based on the study's findings, you can assess the suitability of capsicum plaster according to your priorities.
- If your primary focus is avoiding systemic side effects: The study indicates capsicum plaster is a strong option, as no body-wide effects were observed.
- If you have sensitive skin or a history of skin allergies: You should be mindful of the 7.5% incidence of local reactions and consider testing the plaster on a small area first.
Ultimately, this research positions capsicum plaster as a treatment whose primary risk is a relatively uncommon and localized skin reaction.
Summary Table:
| Adverse Effect Type | Incidence (Active Plaster) | Incidence (Placebo) | Key Finding |
|---|---|---|---|
| Local Skin Reactions | 7.5% (12/160 patients) | 3.1% (5/160 patients) | Redness, itching, or burning at the application site. |
| Systemic Side Effects | 0% | 0% | No body-wide issues (e.g., dizziness, digestive problems) were observed. |
Need a reliable, safe transdermal pain relief solution for your brand?
This study highlights the importance of a favorable safety profile. At Enokon, we are a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharma distributors and brands. Our technical expertise ensures custom R&D and development to create effective products with well-understood risk profiles.
Contact our experts today to discuss how we can develop a safe and effective pain management solution for your market.
Visual Guide
Related Products
- Heat Relief Capsicum Patch for Lower Back Pain Relief
- Capsaicin Chili Medicated Pain Relief Patches
- Natural Herbal Wormwood Patch Pain Plaster
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
People Also Ask
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- How should the Capsicum HOT PATCH Adhesive Patch be stored? Best Practices for Safety & Effectiveness
- What should be done if a dose of the Capsicum HOT PATCH Adhesive Patch is missed? Follow These Steps for Safe Use
- What precautions should be taken when using the Capsicum HOT PATCH Adhesive Patch? Essential Safety Tips
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
















