The critical factors considered during the development of the methylphenidate transdermal patch were a synthesis of pharmaceutical science, material engineering, and patient safety. The process focused on optimizing the patch's adhesive properties for reliable wear, ensuring the chemical stability of the drug through specialized packaging, and incorporating design features like a smaller size and lower drug content to mitigate risks of abuse and accidental exposure.
The central challenge in creating this patch was not merely delivering a drug through the skin, but engineering a complete, self-contained system. Success required balancing therapeutic efficacy with the physical realities of adhesion, chemical stability, and uncompromising patient safety.

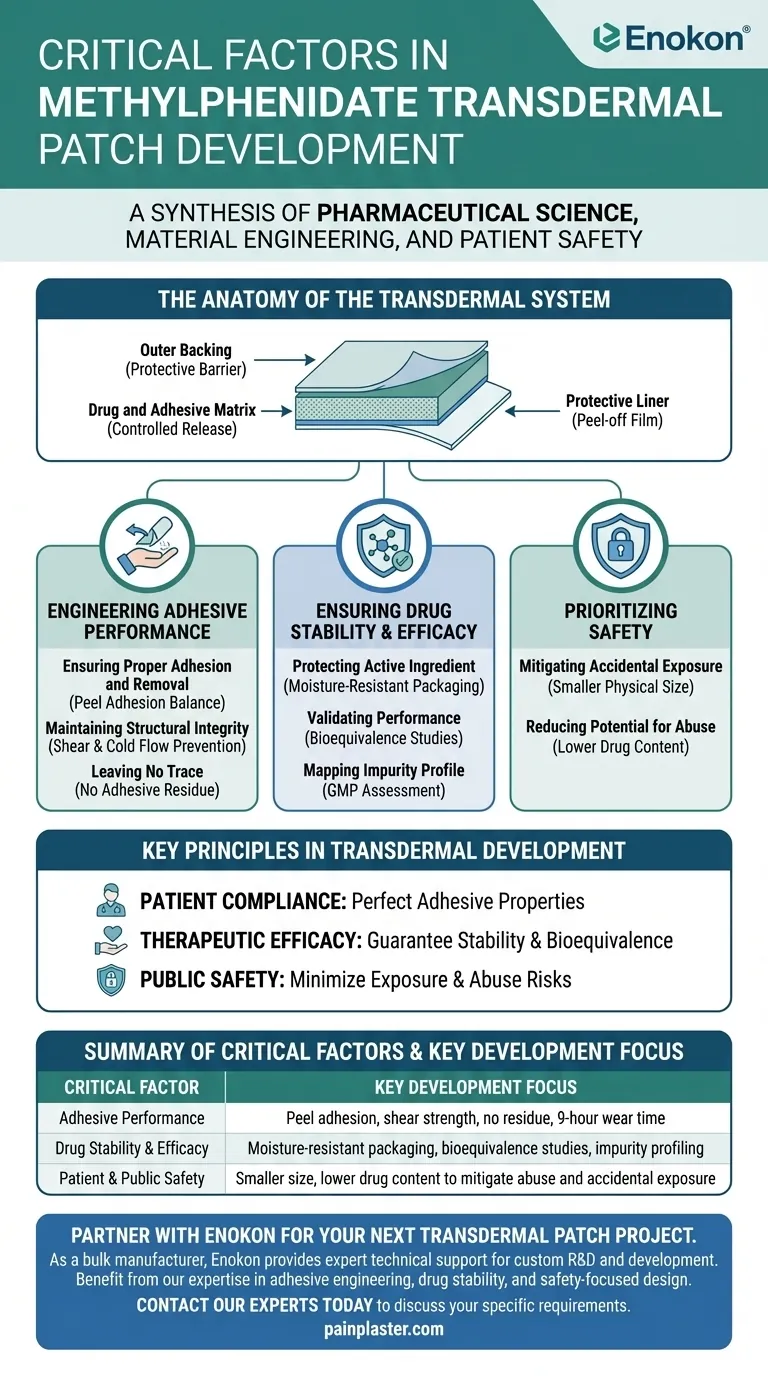
The Anatomy of the Transdermal System
Before examining the development factors, it's essential to understand the patch's basic structure. Each component plays a specific role in the controlled delivery of the medication.
The Protective Liner
This is the film that is peeled off just before application. Its primary function is to protect the adhesive layer and the drug from contamination and degradation until the moment of use.
The Drug and Adhesive Matrix
In this system, the methylphenidate is integrated directly into the adhesive layer. This design requires the adhesive to not only stick to the skin but also to release the drug at a controlled, predictable rate over many hours.
The Outer Backing
This is the visible, outermost layer of the patch. It serves as a protective barrier, shielding the drug matrix from the external environment and preventing the medication from leaking out.
Engineering the Patient Experience: Adhesive Performance
The physical performance of the adhesive is paramount. If the patch fails to adhere properly or causes skin issues, the therapeutic goal is compromised. Rigorous testing ensures it functions correctly throughout its intended 9-hour wear time.
Ensuring Proper Adhesion and Removal
The patch was tested for peel adhesion, which measures the force required to remove it from the skin. The goal is a delicate balance: it must stick securely enough to last all day but be removed without causing excessive pain or skin irritation.
Maintaining Structural Integrity
Shear testing ensures the patch holds together and doesn't slip or slide on the skin with body movement. Developers also engineered it to prevent cold flow, a phenomenon where the adhesive can slowly ooze from the edges, creating a sticky residue and altering the patch's effective surface area.
Leaving No Trace
A key quality metric was ensuring no adhesive residue was left on the skin after removal. This is crucial for patient comfort and simplifies the process of rotating application sites, as instructed for proper use.
Ensuring Drug Stability and Efficacy
Beyond its physical properties, the patch must be a stable and reliable vehicle for the active pharmaceutical ingredient. This involves protecting the drug from degradation and proving its performance in clinical settings.
Protecting the Active Ingredient
Stability studies revealed the importance of controlling the patch's environment. The final design includes moisture-resistant packaging to prevent the chemical degradation of methylphenidate, ensuring the patient receives the correct dose from a stable product.
Validating Performance
The patch underwent extensive clinical pharmacokinetic studies. The primary goal of these studies was to demonstrate bioequivalence, proving that the transdermal system delivers the drug to the bloodstream in a comparable manner to the established oral medication.
Mapping the Impurity Profile
As part of Good Manufacturing Practices, a thorough impurity profile assessment was conducted. This process identifies and quantifies any potential degradation products or contaminants to ensure the product is safe and pure.
Understanding the Trade-offs: Prioritizing Safety
Every design choice involves trade-offs. For the methylphenidate patch, key decisions were made to prioritize safety, sometimes over other factors like manufacturing cost or size.
Mitigating Accidental Exposure
The patch was deliberately designed with a smaller physical size compared to other transdermal systems. This reduces the surface area, which in turn lowers the risk of a child or other individual coming into accidental contact with the medication.
Reducing Potential for Abuse
A lower total drug content was engineered into the patch. While it contains enough medication for its intended therapeutic duration, the reduced amount makes it a less attractive target for extraction or misuse, which is a critical consideration for a controlled substance.
Key Principles in Transdermal Development
The development of this patch highlights a set of core priorities that guide the creation of any effective transdermal system.
- If your primary focus is patient compliance: You must perfect the adhesive's properties for a comfortable, secure, and clean experience.
- If your primary focus is therapeutic efficacy: You must guarantee drug stability, control the release rate, and prove bioequivalence through rigorous clinical trials.
- If your primary focus is public safety: You must intentionally design features like a smaller size and lower drug load to minimize risks of accidental exposure and abuse.
Ultimately, a successful transdermal patch is a testament to a development process that addresses the complex interplay between the drug, the delivery system, and the patient.
Summary Table:
| Critical Factor | Key Development Focus |
|---|---|
| Adhesive Performance | Peel adhesion, shear strength, no residue, 9-hour wear time |
| Drug Stability & Efficacy | Moisture-resistant packaging, bioequivalence studies, impurity profiling |
| Patient & Public Safety | Smaller size, lower drug content to mitigate abuse and accidental exposure |
Partner with Enokon for Your Next Transdermal Patch Project
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon provides healthcare and pharmaceutical distributors and brands with expert technical support for custom R&D and development. Benefit from our deep expertise in adhesive engineering, drug stability, and safety-focused design to bring your product to market efficiently and effectively.
Contact our experts today to discuss your specific requirements.
Visual Guide
Related Products
- Icy Hot Menthol Medicine Pain Relief Patch
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Menthol Gel Pain Relief Patch
People Also Ask
- Is menthol topical safe during pregnancy and breastfeeding? Key Safety Insights
- What are common side effects of menthol patch? Key Risks & Safety Tips
- Are cooling patches reusable? Understanding Single-Use Cooling Solutions
- How does menthol work in the Reliever Patch? Dual-Action Pain Relief Explained
- How does menthol function as a topical analgesic? The Science Behind Cooling Pain Relief













